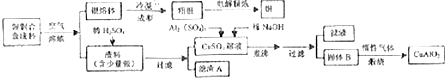
��Ŀ����
3��ij�о���ѧϰС�齫Na2SO3�������������ǿ����600�����ϣ���ȴ���������������ʱ���ԭ������ȡ�������Ⱥ��������ˮ��������ȫ�ܽ⣬������Һʹ��ɫʯ����ֽ��������1����ͬѧ����ʵ���ṩ��Ϣ��������Ϊ��ǿ�Ⱥ������ΪNa2SO3������Ϊ��ͬѧ�����������ǣ��ٹ��������������Һ�ʼ��ԣ�������SO32-ˮ�⣻
��2����ͬѧ��ʵ���ҳ����Լ���������ʵ�鷽���Ʒ��˼�ͬѧ�ķ������ۣ�
��д��Na2SO3��ǿ�ȷ�����Ӧ�Ļ�ѧ����ʽ��4Na2SO3$\frac{\underline{\;600��\;}}{\;}$Na2S+3Na2SO4��
�ڼ�����ͬѧ��ʵ����ƣ�ǿ�Ⱥ�����������Թ��У���ϡ�����г�������ζ�������ɣ�֤��Na2SO3ǿ�ȷֽ��������
���� ��1����ͬѧ��Ϊ��ǿ�Ⱥ������ΪNa2SO3�����ɿ����ǹ������ʱ���ԭ���������ұ����������ƾ���ǿ��������ˮ��ʼ���ʹ��ɫʯ����ֽ������
��2��С�齫Na2SO3�������������ǿ����600�����ϣ����������Ӧ��Ӧ����������������ԭ��Ӧ�����Ҳ��ﶼ�ǹ��壬������������������䣬����һ�����ʻ����ֹ������ʱ����ǿ�������Σ�������ֻ������Ԫ�صĻ��ϼ۵��������ɴ˷������
��� �⣺��1�����������Ϣ����ͬѧ��Ϊ��ǿ�Ⱥ������ΪNa2SO3�����ɿ����ǹ������ʱ���ԭ���������ұ����������ƾ���ǿ��������ˮ��ʼ���ʹ��ɫʯ����ֽ�������ʴ�Ϊ���ٹ��������������Һ�ʼ��ԣ�������SO32-ˮ�⣻
��2����Na2SO3��ǿ�ȷ�����Ӧ��������������ԭ��Ӧ��+4�۵������ߣ����ֽ��ͣ�Ȼ����ݵ�ʧ�����غ��֪��4Na2SO3$\frac{\underline{\;600��\;}}{\;}$Na2S+3Na2SO4���ʴ�Ϊ��4Na2SO3$\frac{\underline{\;600��\;}}{\;}$Na2S+3Na2SO4��
��ֻҪ֤���������������ӣ�Ӧ��ǿ�������ᣬ���Լ����Ὣ��ת��Ϊ���⣨����֤��������������ӣ����ȼ����ᣬ�ټ��Ȼ����������ʵ��Ϊȡǿ�Ⱥ�����������Թ��У���ϡ�����г�������ζ�������ɣ�֤��Na2SO3ǿ�ȷֽ��������
�ʴ�Ϊ��ȡǿ�Ⱥ�����������Թ��У���ϡ�����г�������ζ�������ɣ�֤��Na2SO3ǿ�ȷֽ��������
���� ���⿼��������ɵ�̽����ѧ��Ҫ�����ѧ��֪ʶ�ۺϷ�����Ȼ�������ʵ�������֤����һ�����Ѷȣ�

 �Ǽ�����������ϵ�д�
�Ǽ�����������ϵ�д� â���̸������Ծ�ϵ�д�
â���̸������Ծ�ϵ�д�| A�� | C2H4��C3H6 | B�� | C2H4��C4H8 | C�� | C3H4��C4H8 | D�� | C3H4��C3H6 |
| A�� | 41 | B�� | 115 | C�� | 142 | D�� | 162 |
| A�� | Fe3O4��������ϡHNO3��Fe3O4+8H+=Fe2++2Fe3++4H2O | |
| B�� | ��������Һ�������Һ��ϣ�SiO32-+2H+=H2SiO3�� | |
| C�� | AlCl3��Һ�м�������İ�ˮ��Al3++4OH-=AlO2-+2H2O | |
| D�� | ��NaHCO3��Һ�м������Ca��OH��2��Һ��HCO3-+Ca2++OH-=CaCO3��+H2O |
| A�� | �����ռ�ʱ������ʹ����ͨʯӢ������SiO2+2NaOH=Na2SiO3+H2O | |
| B�� | �״�ȼ�ϵ�أ�KOH���������Һ���ĸ�����Ӧʽ��CH3OH-6e+H2O=CO2+6H+ | |
| C�� | ���ȵ���˿��ˮ�Ӵ��������γɺ�ɫ�����㣺3Fe+4H2O=Fe3O4+4H2 | |
| D�� | ��84����Һ������Ч�ɷ�NaClO���͡�����顱����Ҫ�ɷ����ᣩ���ʹ�÷ų�������ClO-+Cl-+2H+=Cl2��+H2O |
| A�� | S | B�� | Mg | C�� | Cl | D�� | Be |
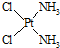 ���е�Pt�Ļ��ϼ�Ϊ+2��
���е�Pt�Ļ��ϼ�Ϊ+2��