��Ŀ����
�����ᣨH3PO3���Ƕ�Ԫ�ᣬH3PO3��Һ���ڵ���ƽ�⣺H3PO3  H+ + H2PO3����������������NaOH��Һ��Ӧ������Na2HPO3��
H+ + H2PO3����������������NaOH��Һ��Ӧ������Na2HPO3��
��1����д��������������NaOH��Һ��Ӧ�����ӷ���ʽ____________________________��
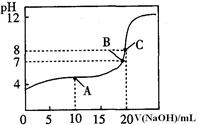
��ij�¶��£�0.1000 mol��L��1��H3PO3��ҺpH�Ķ���Ϊ1.6������ʱ��Һ��c (H+) �� 2.5��10��2 mol��L��1����OH��֮���������ӵ�Ũ����С�����˳���� �����¶���H3PO3����ƽ���ƽ�ⳣ��K= ����H3PO3�ڶ���������Բ��ƣ����������λ��Ч���֣�
����H3PO3��Һ�еμ�NaOH��Һ�����ԣ�������Һ��
c(Na+)_______ c(H2PO3��)+ 2c(HPO32��)��������� ������ ��=������
��2�����������ǿ��ԭ�ԣ���ʹ��ˮ��ɫ���÷�Ӧ�Ļ�ѧ����ʽ_______________________��
��3�����Na2HPO3��ҺҲ�ɵõ������ᣬװ��ʾ��ͼ���£�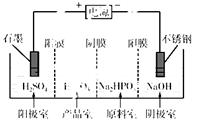
˵������Ĥֻ����������ͨ������Ĥֻ����������ͨ����
�������ĵ缫��ӦʽΪ________________________��
�ڲ�Ʒ���з�Ӧ�����ӷ���ʽΪ________________��
��1�� ��H3PO3+OH����H2PO3��+H2O (2��)
��c(HPO32��)��c(H2PO3��)��c(H+) (2��) 8.3��10��3mol/L(2�֣�д��д��λ����ȷ)
�� �� (2��)
��2��H3PO3+I2+H2O��H3PO4+2HI(2��)
��3����2H++2e����H2�� (2��)
��HPO32��+2H+��H3PO3(2��) ��HPO32��+H+��H2PO3����H2PO3��+H+��H3PO3(��1��)
���������������1�����������Ƕ�Ԫ�ᣬ������������������Ʒ�Ӧ����NaH2PO3��H2O�����Ը÷�Ӧ����ʽΪ��H3PO3+OH-=H2PO3-+H2O���ʴ�Ϊ��H3PO3+OH-=H2PO3-+H2O��
��0.1000mol?L-1��H3PO3��ҺpH�Ķ���Ϊ1.6��������Ũ��С��������Ũ�ȣ������������Ƕ�Ԫ���ᣬ��ˮ�зֲ����룬�ҵ�һ������̶ȴ��ڵڶ��������������ж������������ɣ�����������Ũ���������Ũ�ȴ�С˳����c��HPO32-����c��H2PO3-����c��H+����
H3PO3  H++H2PO3-
H++H2PO3-
��ʼʱ������Ũ�ȣ�mol?L-1�� 0.10 0 0
��Ӧ�ĸ����ʵ�Ũ�ȣ�mol?L-1��2.5��10-2 2.5��10-2 2.5��10-2
ƽ��ʱ�����ʵ�Ũ�ȣ�mol?L-1��0.10-2.5��10-2 2.5��10-22.5��10-2
K��c��H+��c��H2PO3?��/c(c(H3PO3)��2.5��10?2��2.5��10?2/0.10-2.5��10?2=8.3��10-3mol/L��
�ʴ�Ϊ��c��HPO32-����c��H2PO3-����c��H+����8.3��10-3mol/L��
����Һ�����ԣ���C��H+��=C��OH-������Һ�ʵ����ԣ���c��Na+��+C��H+��=C��OH-��+c��H2PO3-��+2c��HPO32-������ΪC��H+��=C��OH-��������c��Na+��=c��H2PO3-��+2c��HPO32-�����ʴ�Ϊ��=��
��2�������ǿ�����ԣ����������ǿ��ԭ�ԣ�����������͵��ܷ���������ԭ��Ӧ�������������ᣬ��Ӧ����ʽΪ��H3PO3+I2+H2O=2HI+H3PO4���ʴ�Ϊ��H3PO3+I2+H2O=2HI+H3PO4��
��3���������������ӵõ��ӷ�����ԭ��Ӧ���缫��ӦʽΪ2H++2e-=H2�����ʴ�Ϊ��2H++2e-=H2����
�ڲ�Ʒ����HPO32-�������ӽ�����������ᣬ��Ӧ���ӷ���ʽΪ��HPO32-+2H+=H3PO3���ʴ�Ϊ��HPO32-+2H+=H3PO3��
���㣺������ʵĵ��롢�缫��Ӧʽ����д���й�ƽ�ⳣ���ļ��㡣

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д���ش��йصζ������е�������⡣
(1)����֪Ũ�ȵ�����������Һ�ζ�δ֪Ũ�ȵ����ᣬ�ζ�������ͼ��ʾ��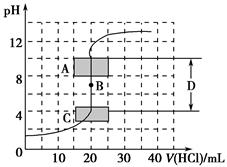
����ͼ��A��pH��Χʹ�õ�ָʾ����________��
C��pH��Χʹ�õ�ָʾ����________��
�����й��������к͵ζ������еIJ�������ȷ����________(�����)��
| A���ü�ʽ�ζ�����ȡ��֪Ũ�ȵ��ռ���Һ |
| B���ζ��ܺ���ƿ�������ô�װҺ��ϴ |
| C���ζ�������ʼ��ע����ƿ����Һ��ɫ�仯 |
| D����ƿ�еĴ���Һ������Ͳ��ȡ |
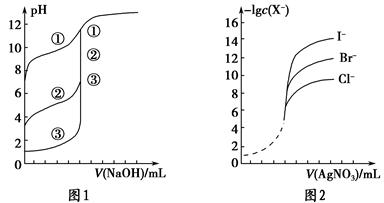
ij�������ĺ����ϴ�����Ҫ��Ni��������Al��Al2O3��Fe��FeO��Fe2O3�������������ʣ������������ʲ�����Ӧ����ijУ��ѧ�о���ѧϰС�����������ͼ��ʾ�ķ������Ըú����ϴ���Ϊԭ�����Ʊ�NiSO4��7H2O��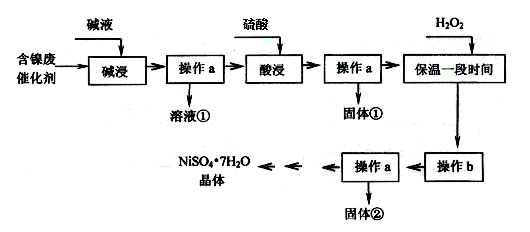
���������ϡ�
��Ni������������ᷴӦ����Ni2+�������Һ��Ӧ��
�ڲ���������������������ʽ����ʱpH���£�
| ������ | Al��OH��3 | Fe��OH��3 | Fe��OH��2 | Ni��OH��2 |
| ��ʼ������pH | 4.1 | 1.7 | 7.6 | 7.0 |
| ��ȫ������pH | 5.2 | 3.2 | 9.5 | 9.2 |
��1������aΪ ��
��2�����������Ŀ���dz�ȥ ���ѧʽ����
��3������bΪ������Һ��pH������ΪpH����ѵ��ط�Χ�� ��
��4��NiSO4��ǿ����Һ���ô������������������Ƶü��������ص缫����NiOOH��
��д���÷�Ӧ�����ӷ���ʽ ��
����֪�����������ܷ�Ӧ�� H2+2NiOOH
 2Ni��OH��2��д���÷�Ӧ�ŵ�ʱ������Ӧʽ ��
2Ni��OH��2��д���÷�Ӧ�ŵ�ʱ������Ӧʽ ����5��һ����Ϊ��������Һ�е�����Ũ��С��1.0��10-5 mol��L-1ʱ�������Ѿ���ȫ���������ϱ������ݹ���Fe��OH��2���ܶȻ�
 ��
�� ��ҵ����ƽ�VOSO4�е�K2SO4��SiO2���ʳ�ȥ�����յõ�V2O5���������£�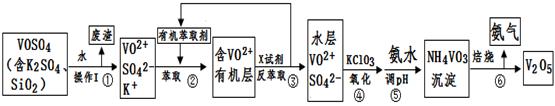
��ش��������⣺
��1����������÷����ijɷ��� ��д��ѧʽ��������I������ ��
��2������ڡ��۵ı仯���̿ɼ�Ϊ����ʽR��ʾVO2+��HA��ʾ�л���ȡ������
R2(SO4)n (ˮ��)+ 2nHA���л��㣩 2RAn���л��㣩 + nH2SO4 (ˮ��)
2RAn���л��㣩 + nH2SO4 (ˮ��)
������ȡʱ��������������ԭ���� ������X�Լ�Ϊ ��
��3���ݵ����ӷ���ʽΪ ��
��4��25��ʱ��ȡ����������������õ��������ʺ���ҺpH֮���ϵ���±���
| pH | 1.3 | 1.4 | 1.5 | 1.6 | 1.7 | 1.8 | 1.9 | 2.0 | 2.1 |
| ��������% | 88.1 | 94.8 | 96.5 | 98.0 | 98.8 | 98.8 | 96.4 | 93.1 | 89.3 |
����������Ϊ93.1%ʱ������Fe(OH)3����������Һ��c(Fe3+)< ��
����֪��25��ʱ��Ksp[Fe(OH)3]=2.6��10-39��
��5���ù��������У�����ѭ�����õ������� �� ��
ij���Ṥ�������Է�ˮ���飨As��Ԫ�غ������ߣ�Ϊ��������ŷţ����û�ѧ���������������ˮ�������������£�
����������±�����ش��������⣺
��1�������ε�Ksp
| ������ | Ksp |
| Ca3(AsO4)2 | 6.8��10��19 |
| CaSO4 | 9.1��10��6 |
| FeAsO4 | 5.7��10��21 |
��2��������Ⱦ���ŷ�Ũ�ȼ������ŷű�
| ��Ⱦ�� | H2SO4 | As |
| ��ˮŨ�� | 29.4g/L | 1.6g��L��1 |
| �ŷű� | pH 6��9 | 0.5mg��L��1 |
��1�������Ṥ���ŷŵķ�ˮ����������ʵ���Ũ��c(H2SO4)= mol��L��1��
��2�������Է�ˮ��Fe3+��Ũ��Ϊ1.0��10��4mol��L��1��c(AsO43��)= mol��L��1��
��3�������ŷų������Է�ˮ�е������飨H3AsO3���ᣩ���׳�������Ͷ��MnO2�Ƚ�������������飨H3AsO4���ᣩ��MnO2����ԭΪMn2������Ӧ�����ӷ���ʽΪ ��
��4���ڴ��������ˮʱ���÷ֶ�ʽ�������ˮ��Ͷ����ʯ�ҵ���pH��2����Ͷ����ʯ�ҽ�pH���ڵ�8����ʹ�������Ca3(AsO4)2��ʽ������
�ٽ�pH���ڵ�2ʱ��ˮ���д�������������������Ҫ�ɷֵĻ�ѧʽΪ ��
��Ca3(AsO4)2��pH���ڵ�8���Ҳſ�ʼ������ԭ��Ϊ
��
�����ᣨH3AsO4���ֲ������ƽ�ⳣ��(25��)Ϊ��K1=5.6��10��3 K2=1.7��10��7 K3=4.0��10��12�������������ƽ�ⳣ���ı���ʽΪK3= ��Na3AsO4�ĵ�һ��ˮ������ӷ���ʽΪ��AsO43��+H2O
 HAsO42��+OH�����ò�ˮ���ƽ�ⳣ����25�棩Ϊ�� ��������λ��Ч���֣���
HAsO42��+OH�����ò�ˮ���ƽ�ⳣ����25�棩Ϊ�� ��������λ��Ч���֣���