��Ŀ����
����Ŀ����֪2A(g)��B(g)![]() 2C(g)����H����a kJ��mol��1(a>0)����һ���д����Ĺ̶��ݻ��������м���2 mol A��1 mol B����500 ��ʱ��ַ�Ӧ��ƽ���C��Ũ��Ϊ�� mol��L��1���ų�������Ϊb kJ��
2C(g)����H����a kJ��mol��1(a>0)����һ���д����Ĺ̶��ݻ��������м���2 mol A��1 mol B����500 ��ʱ��ַ�Ӧ��ƽ���C��Ũ��Ϊ�� mol��L��1���ų�������Ϊb kJ��
��1����֪��A(g)��X(g)![]() 2B(g)����H����133.2 kJ��mol��1��
2B(g)����H����133.2 kJ��mol��1��
5A(g)��X(g)![]() 4C(g)����H����650.4 kJ��mol��1����a��________��
4C(g)����H����650.4 kJ��mol��1����a��________��
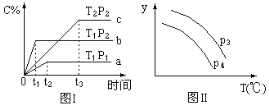
��2����ͬ�¶��¸÷�Ӧ��ƽ�ⳣ�������ʾ���ɴ˿���֪������T1________T2(����>������������<��)��
T/K | T1 | T2 | T3 |
K | 6.86 | 2.45 | 1.88 |
��3����������������Ϊ��ѹ����(��Ӧǰ�����ͬ)����ʼʱ����2 mol A��1 mol B��500 ��ʱ��ַ�Ӧ��ƽ��ų�������Ϊd kJ����d________b(����>������������<��)��
��4����һ���¶��£���һ���ݻ��ɱ�ĺ�ѹ�����У�ͨ��3 mol A��2 mol B��������Ӧ2A(g)��B(g)![]() 2C(g)��ƽ��ʱ�������������ʵ���Ϊ��ʼʱ��80%������ͬһ��Ӧ�¶ȣ�����ͬ�����У�����ʼͶ������Ϊ6 mol A��4 mol B����ƽ��ʱA���������Ϊ________��
2C(g)��ƽ��ʱ�������������ʵ���Ϊ��ʼʱ��80%������ͬһ��Ӧ�¶ȣ�����ͬ�����У�����ʼͶ������Ϊ6 mol A��4 mol B����ƽ��ʱA���������Ϊ________��
���𰸡�258.6<>25%
��������
(1)��A(g)��X(g)![]() 2B(g)����H����133.2 kJ��mol��1����5A(g)��X(g)
2B(g)����H����133.2 kJ��mol��1����5A(g)��X(g)![]() 4C(g)����H����650.4 kJ��mol��1�����ݸ�˹���ɣ�����-���ã�4A(g)��2B(g)
4C(g)����H����650.4 kJ��mol��1�����ݸ�˹���ɣ�����-���ã�4A(g)��2B(g)![]() 4C(g)����H����(650.4-133.2)kJ��mol��1����2A(g)��B(g)
4C(g)����H����(650.4-133.2)kJ��mol��1����2A(g)��B(g)![]() 2C(g)����H����258.6kJ��mol��1����a��258.6���ʴ�Ϊ��258.6��
2C(g)����H����258.6kJ��mol��1����a��258.6���ʴ�Ϊ��258.6��
(2)��Ӧ�Ƿ��ȷ�Ӧ���¶����ߣ�ƽ�������ƶ���ƽ�ⳣ����С�����ݱ������ݿ�֪��T1��T2���ʴ�Ϊ������
(3)ԭƽ���淴Ӧ���У�ѹǿ���ͣ�������������Ϊ��ѹ����(��Ӧǰ�����ͬ)����ͬ�¶�����ʼ����2molA��1molB����ЧΪ��ԭƽ��Ļ���������ѹǿ��ƽ�������������С�ķ����ƶ�����������Ӧ�����ƶ���B��ת���ʱ��Ӧ�ų������������ں��������дﵽƽ��״̬�ų�����������d��b���ʴ�Ϊ������
(4)�跴Ӧ��B�����ʵ���Ϊx��
2A(g)��B(g)![]() 2C(g)
2C(g)
��ʼ(mol) 3 2 0
��Ӧ(mol) 2x x 2x
ƽ��(mol) 3-2x 2-x 2x
��![]() =80%����ã�x=1mol���¶Ȳ���ƽ�ⳣ�����䣬����ͬһ��Ӧ�¶ȣ�����ͬ�����У�����ʼͶ������Ϊ6 mol A��4 mol B���൱��2��ԭ�����ϲ�����������ʵ�������������������ԭ����2����ƽ�ⲻ�ƶ���ƽ��ʱA������������䣬���ƽ��ʱA���������Ϊ
=80%����ã�x=1mol���¶Ȳ���ƽ�ⳣ�����䣬����ͬһ��Ӧ�¶ȣ�����ͬ�����У�����ʼͶ������Ϊ6 mol A��4 mol B���൱��2��ԭ�����ϲ�����������ʵ�������������������ԭ����2����ƽ�ⲻ�ƶ���ƽ��ʱA������������䣬���ƽ��ʱA���������Ϊ![]() ��100%=25%���ʴ�Ϊ��25%��
��100%=25%���ʴ�Ϊ��25%��