��Ŀ����
��15�֣��ش����и�С�⣺
��1����֪ ��ˮ�еĵ��뷽��ʽΪ
��ˮ�еĵ��뷽��ʽΪ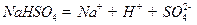 ����
���� ��Һ
��Һ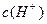 __________
__________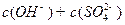 ���>����=����<����1�֣���
���>����=����<����1�֣���
��2�������£���1.0mol/L�� ��Һ����μ�������ʵ���Ũ�ȵ�
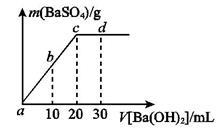
��Һ����μ�������ʵ���Ũ�ȵ� ��Һ�����������������������������Һ�������ϵ��ͼ��ʾ��a��b��c��d�ֱ��ʾʵ��ʱ��ͬ�ε���Һ������b���ʾ��Һ��_________������ԡ������ԡ����ԡ���1�֣���c����ʾ�����ӷ���ʽΪ______________________________________________________��2�֣���
��Һ�����������������������������Һ�������ϵ��ͼ��ʾ��a��b��c��d�ֱ��ʾʵ��ʱ��ͬ�ε���Һ������b���ʾ��Һ��_________������ԡ������ԡ����ԡ���1�֣���c����ʾ�����ӷ���ʽΪ______________________________________________________��2�֣���
��3����T��ʱ���� ������뵽pH=6������ˮ�У������¶Ȳ��䣬�����Һ��pHΪ2��T�潫__________25�棨����ڡ����ڡ���1�֣���K
������뵽pH=6������ˮ�У������¶Ȳ��䣬�����Һ��pHΪ2��T�潫__________25�棨����ڡ����ڡ���1�֣���K Ϊ__________��1�֣����ڸ���Һ����ˮ�������
Ϊ__________��1�֣����ڸ���Һ����ˮ�������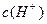 Ϊ__________mol��L-1��1�֣���T��ʱ����pH=11��NaOH��ҺV1L��pH=1��
Ϊ__________mol��L-1��1�֣���T��ʱ����pH=11��NaOH��ҺV1L��pH=1��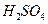 ��ҺV2L��ϣ����Ϻ���Һ�����Ϊԭ����Һ���֮�ͣ������û����Һ��pH=2����V1: V2 =____________��2�֣�������Һ�и������ӵ�Ũ���ɴ�С������˳��Ϊ________________________________________________��2�֣���
��ҺV2L��ϣ����Ϻ���Һ�����Ϊԭ����Һ���֮�ͣ������û����Һ��pH=2����V1: V2 =____________��2�֣�������Һ�и������ӵ�Ũ���ɴ�С������˳��Ϊ________________________________________________��2�֣���
��4��0.1 mol��L-1pHΪ4��NaHB��Һ�Т�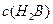 ����
����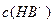 ����
���� �ɴ�С��˳��Ϊ_______________��2�֣���
�ɴ�С��˳��Ϊ_______________��2�֣���
��5����0.1 mol��L-1�Ģ�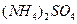 ��Һ����
��Һ���� ��Һ����
��Һ���� ��Һ�У�
��Һ�У�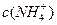 �ɴ�С������˳��Ϊ_______________________��2�֣���
�ɴ�С������˳��Ϊ_______________________��2�֣���
��1����֪
 ��ˮ�еĵ��뷽��ʽΪ
��ˮ�еĵ��뷽��ʽΪ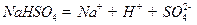 ����
���� ��Һ
��Һ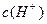 __________
__________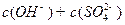 ���>����=����<����1�֣���
���>����=����<����1�֣���
��2�������£���1.0mol/L��
 ��Һ����μ�������ʵ���Ũ�ȵ�
��Һ����μ�������ʵ���Ũ�ȵ� ��Һ�����������������������������Һ�������ϵ��ͼ��ʾ��a��b��c��d�ֱ��ʾʵ��ʱ��ͬ�ε���Һ������b���ʾ��Һ��_________������ԡ������ԡ����ԡ���1�֣���c����ʾ�����ӷ���ʽΪ______________________________________________________��2�֣���
��Һ�����������������������������Һ�������ϵ��ͼ��ʾ��a��b��c��d�ֱ��ʾʵ��ʱ��ͬ�ε���Һ������b���ʾ��Һ��_________������ԡ������ԡ����ԡ���1�֣���c����ʾ�����ӷ���ʽΪ______________________________________________________��2�֣�����3����T��ʱ����
 ������뵽pH=6������ˮ�У������¶Ȳ��䣬�����Һ��pHΪ2��T�潫__________25�棨����ڡ����ڡ���1�֣���K
������뵽pH=6������ˮ�У������¶Ȳ��䣬�����Һ��pHΪ2��T�潫__________25�棨����ڡ����ڡ���1�֣���K Ϊ__________��1�֣����ڸ���Һ����ˮ�������
Ϊ__________��1�֣����ڸ���Һ����ˮ�������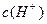 Ϊ__________mol��L-1��1�֣���T��ʱ����pH=11��NaOH��ҺV1L��pH=1��
Ϊ__________mol��L-1��1�֣���T��ʱ����pH=11��NaOH��ҺV1L��pH=1��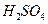 ��ҺV2L��ϣ����Ϻ���Һ�����Ϊԭ����Һ���֮�ͣ������û����Һ��pH=2����V1: V2 =____________��2�֣�������Һ�и������ӵ�Ũ���ɴ�С������˳��Ϊ________________________________________________��2�֣���
��ҺV2L��ϣ����Ϻ���Һ�����Ϊԭ����Һ���֮�ͣ������û����Һ��pH=2����V1: V2 =____________��2�֣�������Һ�и������ӵ�Ũ���ɴ�С������˳��Ϊ________________________________________________��2�֣�����4��0.1 mol��L-1pHΪ4��NaHB��Һ�Т�
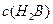 ����
����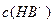 ����
���� �ɴ�С��˳��Ϊ_______________��2�֣���
�ɴ�С��˳��Ϊ_______________��2�֣�����5����0.1 mol��L-1�Ģ�
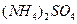 ��Һ����
��Һ���� ��Һ����
��Һ���� ��Һ�У�
��Һ�У�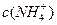 �ɴ�С������˳��Ϊ_______________________��2�֣���
�ɴ�С������˳��Ϊ_______________________��2�֣���(1)=
(2)����

(3)����

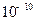 9:11
9:11 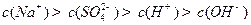
(4)��>��>��
(5)��>��>��>��
�����ۺϿ�����ˮ�ĵ��롢�����ˮ�⡢����Ũ�ȴ�С�ıȽϡ����ӷ���ʽ���� д��ͬʱ��������ҺpH�ļ��㣬Ҫ�������н�ǿ���ۺϷ���������(1)
д��ͬʱ��������ҺpH�ļ��㣬Ҫ�������н�ǿ���ۺϷ���������(1) ��Һ�е���غ㣺
��Һ�е���غ㣺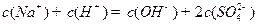 ����
����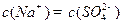 �������
�������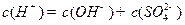 ��(2)ͼ����c��Ϊ�յ㣬˵��
��(2)ͼ����c��Ϊ�յ㣬˵��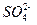 ǡ����ȫ������ͬʱҲ˵����Һ�����Ϊ20 mL�����ӷ���ʽΪ
ǡ����ȫ������ͬʱҲ˵����Һ�����Ϊ20 mL�����ӷ���ʽΪ ����
����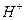 ��
�� ǡ���кͣ���Һ�����ԡ�������10 mL Ba(OH)2��Һʱ����ӦΪ
ǡ���кͣ���Һ�����ԡ�������10 mL Ba(OH)2��Һʱ����ӦΪ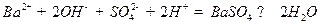 ��(3)��ˮ��pH=6��˵��
��(3)��ˮ��pH=6��˵��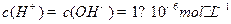 ��
��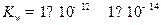 ��˵���¶ȸ���25�档
��˵���¶ȸ���25�档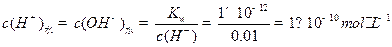 ��pH=11��NaOH��Һ
��pH=11��NaOH��Һ
 ��������ã�
��������ã�
 ����ã�
����ã� :
: =9:11�������Һ�и�����Ũ�ȴ�С��ϵΪ
=9:11�������Һ�и�����Ũ�ȴ�С��ϵΪ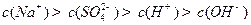 ��(4)��0.1
��(4)��0.1 ��NaHB��Һ��pHΪ4������
��NaHB��Һ��pHΪ4������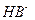 �ĵ���̶ȴ�����ˮ��̶ȣ��������Ũ�ȴ�С��ϵΪ��>��>�١�(5)��ͬ����Һ������Ũ�ȴ�С�Ƚϵ�һ�㷽�����ȿ����룬����Ķ࣬Ũ�ȴ��������ͬʱ���ٿ�ˮ��̶ȵĴ�С��
�ĵ���̶ȴ�����ˮ��̶ȣ��������Ũ�ȴ�С��ϵΪ��>��>�١�(5)��ͬ����Һ������Ũ�ȴ�С�Ƚϵ�һ�㷽�����ȿ����룬����Ķ࣬Ũ�ȴ��������ͬʱ���ٿ�ˮ��̶ȵĴ�С��
�������������Һ��pHʱ��һ��Ҫ����ˮ�����ӻ������¶Ȳ���25��ʱ��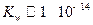 ������ʱ���ע�⣬�����׳�����
������ʱ���ע�⣬�����׳�����
 д��ͬʱ��������ҺpH�ļ��㣬Ҫ�������н�ǿ���ۺϷ���������(1)
д��ͬʱ��������ҺpH�ļ��㣬Ҫ�������н�ǿ���ۺϷ���������(1) ��Һ�е���غ㣺
��Һ�е���غ㣺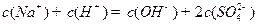 ����
����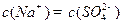 �������
�������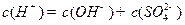 ��(2)ͼ����c��Ϊ�յ㣬˵��
��(2)ͼ����c��Ϊ�յ㣬˵��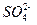 ǡ����ȫ������ͬʱҲ˵����Һ�����Ϊ20 mL�����ӷ���ʽΪ
ǡ����ȫ������ͬʱҲ˵����Һ�����Ϊ20 mL�����ӷ���ʽΪ ����
����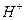 ��
�� ǡ���кͣ���Һ�����ԡ�������10 mL Ba(OH)2��Һʱ����ӦΪ
ǡ���кͣ���Һ�����ԡ�������10 mL Ba(OH)2��Һʱ����ӦΪ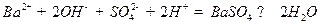 ��(3)��ˮ��pH=6��˵��
��(3)��ˮ��pH=6��˵��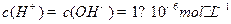 ��
��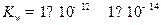 ��˵���¶ȸ���25�档
��˵���¶ȸ���25�档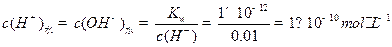 ��pH=11��NaOH��Һ
��pH=11��NaOH��Һ ��������ã�
��������ã� ����ã�
����ã�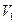 :
: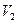 =9:11�������Һ�и�����Ũ�ȴ�С��ϵΪ
=9:11�������Һ�и�����Ũ�ȴ�С��ϵΪ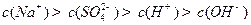 ��(4)��0.1
��(4)��0.1 ��NaHB��Һ��pHΪ4������
��NaHB��Һ��pHΪ4������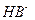 �ĵ���̶ȴ�����ˮ��̶ȣ��������Ũ�ȴ�С��ϵΪ��>��>�١�(5)��ͬ����Һ������Ũ�ȴ�С�Ƚϵ�һ�㷽�����ȿ����룬����Ķ࣬Ũ�ȴ��������ͬʱ���ٿ�ˮ��̶ȵĴ�С��
�ĵ���̶ȴ�����ˮ��̶ȣ��������Ũ�ȴ�С��ϵΪ��>��>�١�(5)��ͬ����Һ������Ũ�ȴ�С�Ƚϵ�һ�㷽�����ȿ����룬����Ķ࣬Ũ�ȴ��������ͬʱ���ٿ�ˮ��̶ȵĴ�С���������������Һ��pHʱ��һ��Ҫ����ˮ�����ӻ������¶Ȳ���25��ʱ��
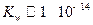 ������ʱ���ע�⣬�����׳�����
������ʱ���ע�⣬�����׳�����
��ϰ��ϵ�д�
 Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д�
Ӯ�ڿ�����ʦ��ʱ�ƻ�ϵ�д� �������Ͽ�ʱͬ��ѵ��ϵ�д�
�������Ͽ�ʱͬ��ѵ��ϵ�д�
�����Ŀ