题目内容
工业上生产氯气常用电解槽中电解饱和食盐水,为了避免电解产物之间发生反应,常用阳离子交换膜将电解槽隔成两部分。下图(左图)为电解槽的示意图。
(1)这种阳离子交换膜,只允许溶液中的 通过。(填下列微粒的编号)
①H2 ②Cl2 ③H+ ④Cl- ⑤Na+ ⑥OH-
(2)写出在电解过程中发生的电极方程式阳极: 。
(3)已知某电解槽每小时加入10%的氢氧化钠溶液10kg,每小时能收集到标况下的氢气896L,而且两边的水不能自由流通。则理论上计算,电解后流出的氢氧化钠溶液的质量分数为 。
(4)某化学课外兴趣小组设计了用电解法制取乙醇钠的工业方法,所用的电解槽如上右图所示,设计要求:①所用的交换膜不能让分子自由通过;②电解过程中消耗的原料是氢氧化钠和乙醇。回答下列问题:
①写出在电解过程中发生的电极方程式
阴极: 。
②最后从乙醇钠的乙醇溶液中分离得到纯净乙醇钠固体的方法是: 。
(1)③⑤(3分)(2)阳极;2Cl--2e-=Cl2(3分) (3)35.7%(3分)
(4)①阴极:2CH3CH2OH+2e-=2CH3CH2O-+H2(3分)②蒸发结晶 (3分)
解析试题分析:(1)阳离子交换膜只允许阳离子钠离子、氢离子通过,而阴离子和分子均不能通过,故答案为:③⑤。
(2)电解池中和电源的正极相连的是电解池的阳极,阳极上氯离子发生失电子的氧化反应,即2Cl--2e-=Cl2↑,故答案为:2Cl--2e-=Cl2↑。
(3)氢气的物质的量=896L÷22.4L/mol=40mol,质量为40mol×2g/mol=80g,根据电解方程式:2NaCl+2H2O  2NaOH+H2↑+Cl2↑,可得生成的NaOH为:m(NaOH)=2×40mol×40g/mol=3200g,溶液增加的质量为增加的钠元素的质量减去生成氢气的质量,为:2×40mol×23g/mol-80g=1760g;溶液中溶质氢氧化钠的质量为10000g×10%+3200g=4200g,溶液的质量为10000g+1760g=11760g,电解后流出的氢氧化钠溶液的质量分数=4200g÷11760g×100%=35.7%。
2NaOH+H2↑+Cl2↑,可得生成的NaOH为:m(NaOH)=2×40mol×40g/mol=3200g,溶液增加的质量为增加的钠元素的质量减去生成氢气的质量,为:2×40mol×23g/mol-80g=1760g;溶液中溶质氢氧化钠的质量为10000g×10%+3200g=4200g,溶液的质量为10000g+1760g=11760g,电解后流出的氢氧化钠溶液的质量分数=4200g÷11760g×100%=35.7%。
(4)①电解池中和电源的负极相连的是电解池的阴极,阴极上乙醇得电子发生还原反应,即2CH3CH2OH+2e-=2CH3CH2O-+H2↑。
②从溶液中析出溶质的方法是蒸发结晶,故答案为:蒸发结晶.
考点:本题考查电解原理,物质的分离、提纯和除杂、电解方程式的书写及相关计算。

 智慧小复习系列答案
智慧小复习系列答案海洋资源的开发与利用具有广阔的前景。海水的pH一般在7.5~8.6之间。某地海水中主要离子的含量如下表:
| 成分 | Na+ | K+ | Ca2+ | Mg2+ | Cl- | S | HC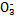 |
| 含量/(mg·L-1) | 9 360 | 83 | 200 | 1 100 | 16 000 | 1 200 | 118 |
(1)海水显弱碱性的原因是(用离子方程式表示): ,该海水中Ca2+的物质的量浓度为 mol·L-1。
(2)电渗析法是近年发展起来的一种较好的海水淡化技术,其原理如下图所示。其中阴(阳)离子交换膜只允许阴(阳)离子通过。
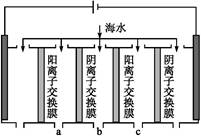
电渗析原理示意图
①阴极的电极反应为 。
②电解一段时间,阴极区会产生水垢,其成分为CaCO3和Mg(OH)2,写出生成CaCO3的离子方程式: 。
③淡水的出口为a、b、c中的 出口。
 (1)工业上可利用反应Na(1)+ KCl(1) K(g)+ NaCl(1)来治炼金属钾,此反应利用了钠的还原性及___ _____,
(1)工业上可利用反应Na(1)+ KCl(1) K(g)+ NaCl(1)来治炼金属钾,此反应利用了钠的还原性及___ _____,





 沉淀完全,而在pH≥3.2时
沉淀完全,而在pH≥3.2时

 为d,则此时的铬酸钾的转化率为 。
为d,则此时的铬酸钾的转化率为 。