��Ŀ����
19���١�����������Ԫ�ؾ���Ԫ�����ڱ�ǰ4���ڣ���ԭ�Ӱ뾶������˳��������У����ǵ���Ҫ���ϼ������ʾ�����Т��ǹ��ɽ�������M��������δ�ɶԵ�����Ϊ1������Ϊ����Ԫ�أ�| ���� | �� | �� | �� | �� | �� | �� | �� | �� | �� |
| �뾶��pm�� | 30 | 64 | 66 | 70 | 106 | 108 | 128 | 186 | 232 |
| ��Ҫ���ϼ� | +1��-1 | -1 | -2 | -3��+5 | -2��+4��+6 | -3��+5 | +2 | +1 | +1 |
��2��Ԫ�آݵ��⻯��Ŀռ乹����V�ͣ�Ԫ�آ��⻯������ԭ�ӵ��ӻ���ʽ��sp3��Ԫ�آܢ��⻯��ķе��ɸߵ��͵�˳����NH3�������ʽ���������ǰ�����֮���������
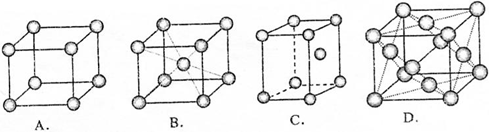
��3��Ԫ�آᵥ�ʾ����ԭ�Ӷѻ���ʽ��B��
��4���ߵĻ�̬ԭ�Ӻ�������Ų�ʽΪ1s22s22p63s23p63d104s1�����������Ρ��Ȼ���廯������������ˮ�Ժ�����Ƶ���ɫ��ԭ��������Щ��Һ�ж�������ͬ����������[Cu��H2O��4]2+��
��5������Ԫ�آ٢ۢݢ���ɵ����ֻ�����������ӷ�Ӧ����ʽ��HSO3-+H+=H2O+SO2����
���� ԭ�ӵĵ��Ӳ���Խ�࣬��ԭ�Ӱ뾶Խ��ͬһ����Ԫ���У�ԭ�Ӱ뾶����ԭ���������������С��Ԫ�ص������=������������-8���������=������������OԪ��û��������ۣ�Fֻ��-1�ۣ�H��+1��-1���ּ�̬��ͬ����Ԫ�ص�ԭ�ӣ������ң�ԭ�Ӱ뾶��С��ͬ����Ԫ�ص�ԭ�ӣ����ϵ��£�ԭ�Ӱ뾶�������ݻ��ϼ�ȷ��Ԫ�أ����ǹ��ɽ�������M��������δ�ɶԵ�����Ϊ1�����ΪCu���ٸ���Ԫ���뻯����Ľṹ���������ش�
��� �⣺����Ԫ�صĻ��ϼ۹��ɣ�Ԫ�ص������=������������-8���������=������������OԪ��û��������ۣ�Fֻ��-1�ۣ�H��+1��-1���ּ�̬������ԭ�Ӱ뾶�Ĺ�ϵ��ͬ����Ԫ�ص�ԭ�ӣ������ң�ԭ�Ӱ뾶��С��ͬ����Ԫ�ص�ԭ�ӣ����ϵ��£�ԭ�Ӱ뾶�����ó��١��ޡ����ֱ���H��F��O��N��S��P��Na��K�����ǹ��ɽ�������M��������δ�ɶԵ�����Ϊ1�����ΪCu��
��1��FԪ�������ڱ��е�λ���ǵڶ����ڵڢ���A�壬F��O��N�У�ͬ���ڴ������ҵ�һ����������һ������F��O������NԪ�ص��������Ӵ��ڰ���״̬����һ���ȶ��ṹ�����Ե�һ������N��O����һ������O��С��
�ʴ�Ϊ���ڶ����ڵڢ���A�壻O��
��2��H2S�У���ԭ�Ӽ۲���Ӷ���Ϊ$\frac{6+2}{2}$=4����ԭ�������Թµ��Ӷԣ���������Ŀռ乹��ΪV�ͣ�PH3�У���ԭ�Ӽ۲���Ӷ���Ϊ$\frac{5+3}{2}$=4��������ԭ�Ӱ�sp3��ʽ�ӻ�����NH3��PH3�У����ڰ�����֮����������������ķе�������⣬
�ʴ�Ϊ��V�ͣ�sp3��NH3��������֮���������
��3��������Ϊ���������ṹ����ԭ��λ�ھ��������ĺͶ��㣬
��ѡB��
��4����ΪCu���ߵĻ�̬ԭ�Ӻ�������Ų�ʽΪ1s22s22p63s23p63d104s1�����������Ρ��Ȼ���廯������������ˮ�Ժ�����Ƶ���ɫ��ԭ��������Щ��Һ�ж�����ˮ��ͭ���ӣ����ӷ���Ϊ[Cu��H2O��4]2+��
�ʴ�Ϊ��1s22s22p63s23p63d104s1��[Cu��H2O��4]2+��
��5������Ԫ�آ٢ۢݢ���ɵ����ֻ�����Ϊ�������ƺ����������ƣ����Ƿ��������ӷ�Ӧ����ΪHSO3-+H+=H2O+SO2����
�ʴ�Ϊ��HSO3-+H+=H2O+SO2����
���� ���⿼��ѧ��Ԫ�����ڱ��Ľṹ��Ԫ�������ɡ����ʵ����ʵȷ�����й�֪ʶ��ע��֪ʶ�Ĺ��ɺ������ǽ���ؼ������ɵİ���һ��Ҫ���塢��ȷ���Ѷȴ�

| A�� | Na��H2O | B�� | Na2O��H2O | C�� | Na2O2��CO2 | D�� | Na2O2��H2O |
| A�� | HCl Cl2 | B�� | H2 HCl | C�� | CO2 Cl2 | D�� | HCl CO2 |
| A�� | 2HI��g��?H2��g��+I2��g����H��0 | B�� | N2��g��+3H2��g��?2NH3��g����H��0 | ||
| C�� | C��s��+H2O��g��?CO��g��+H2��g����H��0 | D�� | CaCO3��s��?CO2��g��+CaCO3��s����H��0 |
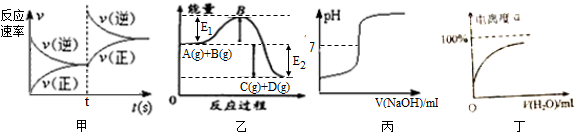
| A�� | ͼ���еķ�Ӧ�ɱ�ʾΪA��g��+B��g��=C��g��+D��g������H=-��E1+E2��KJ/mol | |
| B�� | ͼ�ҿɱ�ʾ�ı�ij��ѧƽ����ϵ���¶�ʱ����Ӧ������ʱ��ı仯��� | |
| C�� | ͼ����ʾ��ϡ�����еμ�����������Һʱ����ҺpH�ı仯��� | |
| D�� | ͼ����ʾij�����������ϡ�������ȵı仯��� |
| A�� | 12C��14C��Ϊͬλ�� | |
| B�� | ��CH3��2CHCH2CH3��CH3C��CH3��3��Ϊͬ���칹�� | |
| C�� | ���ɱ���Ϊͬ�������� | |
| D�� | �Ҵ���CH3CH2OH��������ѣ�CH3-O-CH3����Ϊͬ���칹�� |
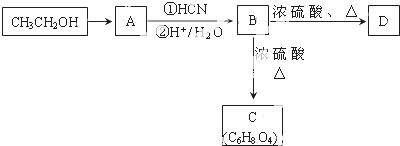
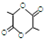 ��
��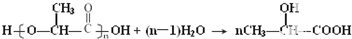 ��
��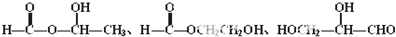 ��
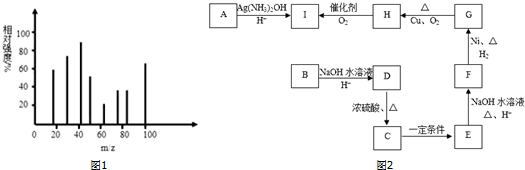
��
 ��CH2��OH��CH2CH2CH2COOH��
��CH2��OH��CH2CH2CH2COOH��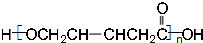 ��
��