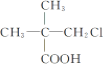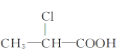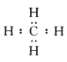��Ŀ����
����Ŀ����Ҫ����д���ƻ�ṹ��ʽ��
(1)3-��-2-������1����ϩ�ṹ��ʽΪ________��
(2)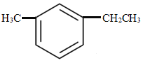 ϵͳ����Ϊ________��
ϵͳ����Ϊ________��
(3)![]() ��ϵͳ����________��
��ϵͳ����________��
(4)��Է�������Ϊ72��֧�����������Ľṹ��ʽ______________����
(5)ij�������ϣԪ�ط���ʵ����̼����������Ϊ85.71%�������������Ϊ14.29%������������ͼ��ʾ����Է�������Ϊ70�������ĺ˴Ź�������ֻ��һ���壬��ͨ������ȷ��������ʵ��ʽ________������ʽ________���ṹ��ʽ________��
���𰸡�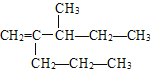 1������3���һ��� 4-��-2-�һ�-1-��ϩ
1������3���һ��� 4-��-2-�һ�-1-��ϩ 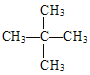 CH2 C5H10
CH2 C5H10 ![]()
��������
�л�����������裺����������ѡ�������������д���ƣ����й����ŵ�д�������ŵ�λ�ã�����ϵͳ����ԭ�������1��-��3����
��4������������ͨʽ���ͬ���칹�����д����
��5����������������л����к��е�C��Hԭ�Ӹ����ȣ��õ�ʵ��ʽ���ٸ�����Է��������������ʽ����Ϻ˴Ź������ĸ���������ṹ��ʽ��
��1��3-��-2-������1����ϩ����ϩ����������5��̼ԭ�ӣ�̼̼˫����1��C��2��C֮�䣬��3��C����һ������2��C����һ�����������л���Ľṹ��ʽΪ��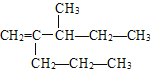 ��
��
��2��![]() Ϊ����ͬϵ�ȡ����λ�ôӼ�ȡ������ʼ��ţ��ʼ����һ��ֱ�λ��1��3��λ�����л��������Ϊ��1������3���һ�����
Ϊ����ͬϵ�ȡ����λ�ôӼ�ȡ������ʼ��ţ��ʼ����һ��ֱ�λ��1��3��λ�����л��������Ϊ��1������3���һ�����
��3��![]() ����ϩ���������������Ϊ
����ϩ���������������Ϊ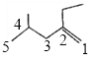 ����ȡ������ǰ������ȡ�����ں�������Ϊ4-��-2-�һ�-1-��ϩ��
����ȡ������ǰ������ȡ�����ں�������Ϊ4-��-2-�һ�-1-��ϩ��
��4������������ͨʽCnH2n��2��֪��Է�������Ϊ72�������ķ���ʽΪC5H12������֧�����Ľṹ��ʽ ��
��
��5���ڸ��л����к��е�C��Hԭ�Ӹ������ǣ�C��H=��85.71%��12����(14.29%��1)=0.071425��0.1429=1:2�����Ը����ʵ�ʵ��ʽ��CH2��ʵ��ʽ��ʽ����14�������ʵ���Է�������Ϊ70��������һ�������к��е����ʽ�ĸ����ǣ�70��14=5���ʸ����ʵķ���ʽ��C5H10�������ĺ˴Ź�������ֻ��һ���壬˵��ֻ����һ��Hԭ�ӣ����ǻ����飬�ṹ��ʽ��![]() ���ʴ�Ϊ��CH2��C5H10��
���ʴ�Ϊ��CH2��C5H10��![]() ��
��

 ÿ�α���ϵ�д�
ÿ�α���ϵ�д�����Ŀ��˫��ˮ��ҽ�ơ����º�ҵ����;�㷺����ҵ�Ϻϳ�˫��ˮ�ķ����ж��֣�����һ�ֺϳɹ���Ϊ�һ�������EAQ������
��֪��
�ٷ�Ӧԭ��Ϊ
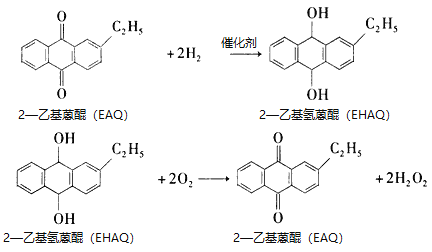
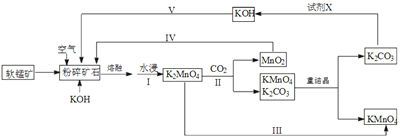
�ڹ�ҵ�Ʊ�������ͼ��ʾ��
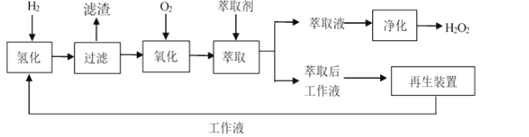
���Ʊ������У���EAQ�����л��ܼ������Ƴɹ���Һ��
��ش��������⣺
��1���������У�ѭ��ʹ�õ�ԭ��Ϊ___��
��2�����мס��ҡ��������л��ܼ���������ʵ��ܽ���������ʾ�������ƹ���Һʱ��Ӧѡ���л��ܼ�__����������������������������ԭ��Ϊ___��
���� | �� | �� | �� |
EAQ | ���� | ���� | ���� |
EHAQ | ���� | ���� | ���� |
H2O2 | ���� | ���� | ���� |
H2O | ���� | ���� | ���� |
��3����������ʱ�������¶�Ϊ45��55����ԭ��Ϊ___��
��4������ȡ��ʱ����ѡ�õ���ȡ����һ�ֳ������ܼ����仯ѧ����Ϊ__��ѡ�ø��ܼ���Ϊ��ȡ����ԭ��Ϊ__��
��5������������Ŀ���ǽ���ȡҺ�еĹ�����������������Ӧѡ�õķ��뷽��Ϊ___��
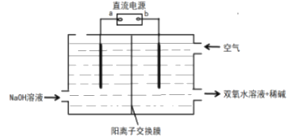
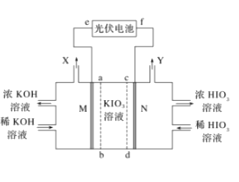
��6�����õ�ⷨ�Ʊ�˫��ˮ��װ��ʾ��ͼ��ͼ��ʾ��ͨ������ĵ缫�ĵ缫��Ӧʽ___��