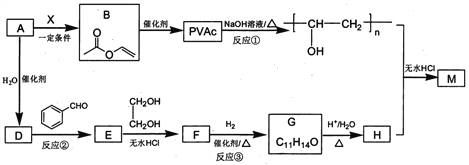
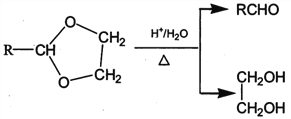
��Ŀ����
����Ŀ��������C��H��O���л���3.24 g��װ��Ԫ�ط���װ�ã�ͨ��������O2ʹ����ȫȼ�գ������ɵ���������ͨ���Ȼ��Ƹ����A�ͼ�ʯ�Ҹ����B�����A������������2.16g��B��������9.24g����֪���л������Է�������Ϊ108��
(1)ȼ�մ˻�����3.24g��������������������______________________��
(2)�û�����ķ���ʽΪ____________��
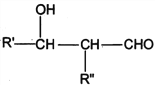
(3)�û�����1�����д���1�����������ں˴Ź�������ͼ����4�����շ塣��д�������ܵĽṹ��ʽ_______________��_______________��
���𰸡�8.16g C7H8O ![]()
![]()
��������
(1)A������������2.16gΪ����ˮ��������B��������9.24gΪ���ɶ�����̼�����������������غ��������������������
(2)�����л��ˮ��������̼�����ʵ���������ԭ���غ�����л��������C��Hԭ����Ŀ�������Է�������ȷ��Oԭ����Ŀ��
(3)�л��ﺬ�б������˴Ź������״����ĸ��壬˵������4��Hԭ�ӣ���Ϸ���ʽ��д���ܵĽṹ��
(1)�Թ�A��������2.16gΪ����ˮ��������B�ܼ�ʯ����CO2����9.24g���������غ㶨�ɣ���֪��������������m(O2)=2.16g+9.24g-3.24g=8.16g��
(2)�Թ�A��������2.16gΪ����ˮ����������ˮ�����ʵ���n(H2O)=2.16g��18g/mol=0.12mol����ʯ�Ҹ����B��������CO2������Ϊ9.24g��������̼�����ʵ���n(CO2)=9.24g��44g/mol=0.21mol�����л�������Ϊ3.24g����Է���������108�������л�������ʵ���Ϊn(�л���)=3.24g��108g/mol=0.03mol�������л�������У�N(C)=![]() =7�� N(H)=
=7�� N(H)=![]() =8�� N(O)=
=8�� N(O)=![]() =1����˸��л���ķ���ʽΪ��C7H8O��
=1����˸��л���ķ���ʽΪ��C7H8O��
(3)�����л��ﺬ�б������Һ˴Ź������״����ĸ��壬���ܵĽṹ��ʽΪ��![]() ��
��![]() ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��ijѧ��������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�����������Һ��
��1�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼ��ʾ����������������Ϊ__mL���ζ��յ�ʱ�������ǣ����������һ������ʱ����ƿ����Һ��ɫ��___����30s���ָ�ԭɫ��

��2��ijѧ����������ʵ��ֱ��¼�й��������±���
�ζ����� | ������������ ��Һ�����/mL | 0.1000mol��L��1��������/mL | ||
�ζ�ǰ�̶� | �ζ���̶� | ��Һ��� | ||
��һ�� | 25.00 | 0.00 | 26.11 | 26.11 |
�ڶ��� | 25.00 | 1.56 | 30.30 | 28.74 |
������ | 25.00 | 0.22 | 26.31 | 26.09 |
��ѡ�����к����������������������Һ���ʵ���Ũ��(����������4λ��Ч����)��c(NaOH)=__mol��L��1��
��3�����ڴ��������ʹ��������������������Һ��Ũ��ƫ�ߵ���__(����ĸ)��
A.�ζ�ǰ�ζ����������ݣ��ζ�����ʧ B.��ʽ�ζ�����ȡNaOH��Һʱ��δ������ϴ����
C.�ζ�ʱ�ﵽ�ζ��յ�ʱ���Ӷ��� D.��ƿȡ��NaOH����Һǰ������ˮϴ��
����Ŀ����������ʵ��������������ó��Ľ�����ȷ����
ѡ�� | ʵ����������� | �� �� |
A | ��һ��Ũ�ȵ�Na2SiO3 ��Һ��ͨ������CO2 ���壬 ���ְ�ɫ������ | H2SiO3 �����Ա�H2CO3������ǿ |
B | ������Fe(NO3)2��ˮ�ܽ�μ�ϡ�����ữ���ٵμ�KSCN��Һ����Һ���Ѫ��ɫ | Fe(NO3)2�ѱ��� |
C | �����£���ã�0.1mol��L-1 Na2SO3��Һ��pHԼΪ10��0.1mol��L-1 NaHSO3��Һ��pHԼΪ5�� | HSO3�� ���H+ ��������SO32����ǿ |
D | �ֱ���25mL��ˮ��25mL��ˮ�е���6��FeCl3 ������Һ��ǰ��Ϊ��ɫ������Ϊ���ɫ�� | �¶����ߣ�Fe3+��ˮ��̶����� |
A.AB.BC.CD.D