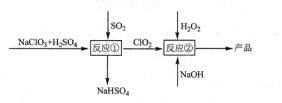
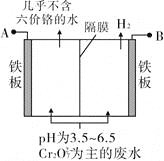
��Ŀ����
����Ŀ����֪�������ݣ�
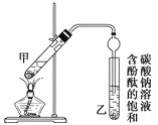
ijѧ����ʵ������ȡ������������Ҫ�������£�
������2 mLŨ���ᡢ3 mL�Ҵ�(��18O)��2 mL����Ļ����Һ��
�ڰ�ͼ���Ӻ�װ��(װ������������)��������Һ����С����ȼ���3��5 min��
�۴��Թ����ռ���һ���������ֹͣ���ȣ������Թ��Ҳ�������Ȼ���ô��ֲ㡣
�ܷ��������������ϴ�ӡ����
(1)���Ƣ��л����Һ�ķ���Ϊ____________����Ӧ��Ũ�����������________________��д����ȡ���������Ļ�ѧ����ʽ��____________��
(2)����ʵ���б���̼������Һ��������(����ĸ)_________��
A���к�������Ҵ� B���к����Ტ�����Ҵ�
C�����������������ܽ� D�������������ɣ���������
(3)���������ҪС����ȼ��ȣ�����Ҫ������_______����������۲쵽��������_________���������Թ��е����ʷ����Եõ���������������ʹ�õ�������________������ʱ����������Ӧ������________________(�����¿ڷ��������Ͽڵ���)����
(4)��ͬѧ����ʵ�飬�ó��Ҵ�������������͵õ��������������������±���
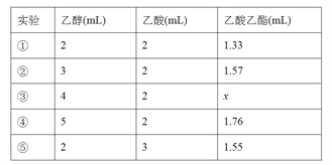
��������x�ķ�Χ��__________________��ʵ��٢ڢ�̽������_____________��
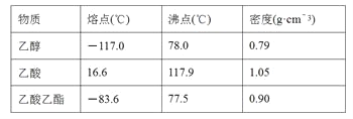
���𰸡���ŨH2SO4�����Ҵ��У��ӱ���Ȼ���������(���Ƚ��Ҵ��������Ϻ��ټ�Ũ���Ტ�ڼ�������в�����) ��������ˮ�� CH3COOH��CH3CH2![]() OH
OH![]() CH3CO18OCH2CH3��H2O BC �����Ȼᵼ�´�����ԭ����������ʧ Һ��ֲ㣬�ϲ�Ϊ��ɫ����ζҺ�壬�²�Ϊdz��ɫҺ�壬���²�Һ����ɫ��dz ��Һ©�� �Ͽڵ� 1.57��1.76 �����Ҵ�����������������IJ�����Ӱ��
CH3CO18OCH2CH3��H2O BC �����Ȼᵼ�´�����ԭ����������ʧ Һ��ֲ㣬�ϲ�Ϊ��ɫ����ζҺ�壬�²�Ϊdz��ɫҺ�壬���²�Һ����ɫ��dz ��Һ©�� �Ͽڵ� 1.57��1.76 �����Ҵ�����������������IJ�����Ӱ��
��������
(1).Ũ������ܶȴ����Ҵ������ᣬ���ʱҪ����������Ҵ��������У�������Ӧ�Ļ����������ǻ������⣬�����ɵ����к���18O��
(2)����̼������Һ�ɳ�ȥ�������������е����ᣬ�ܽ��Ҵ������������������ܽ�Ȳ�������Һ��ֲ㡣
(3)���ᡢ�Ҵ��ӷ��������������ܶȱ�ˮС����״Һ�壬������ˮ������ζ���÷�Һ�����뻥�����ܵ�Һ�壻
(4)�����Ҵ�������ƽ�������ƶ����ڢ�������������1mL �Ҵ������
(1)���ʱŨ�����൱�ڱ�ϡ�ͣ���Ӧ��ŨH2SO4�����Ҵ��У�Ȼ��������ᣬҲ���Ƚ��Ҵ��������Ϻú��ټ���Ũ�����������Ӧ��������Ϊ���淴Ӧ��ʹ��Ũ����ɼӿ�������Ӧ�����ʣ�Ũ���������ˮ�ԣ���������ƽ�����������ķ����ƶ������ᡢ�Ҵ���Ũ������������������������ˮ������������Ӧ�Ļ����������ǻ������⣬�����ɵ����к���18O����Ӧ�ķ���ʽ��CH3COOH��CH3CH2![]() OH
OH![]() CH3CO18OCH2CH3��H2O��
CH3CO18OCH2CH3��H2O��
(2) ����̼������Һ�ɳ�ȥ�������������е����ᣬ�ܽ��Ҵ������������������ܽ�Ȳ�������Һ��ֲ㣬��ѡBC��
(3)�ɱ�������֪�Ҵ��ķе�(78.0 ��)�����������ķе�(77.5 ��)�ܽӽ������ô����ȣ��������Ҵ��ᱻ��������������ԭ�ϵĴ�����ʧ�������ܶ�С��ˮ���ܶȣ����ϲ�Ϊ��״����ζ����ɫҺ�壬����һ��������������������������Na2CO3��Ӧ�����²�Һ���ɫ��dz�����ֲ��Һ����뿪����ʹ�÷�Һ©������Һʱ�ϲ�Һ��Ӧ���Ͽڵ�����
(4) �����Ҵ�������ƽ�������ƶ�����������x�ķ�Χ��1.57��1.76���ڢ�������������1mL �Ҵ������ᣬʵ��٢ڢ�̽�������Ҵ������������ĸı�������ʵ�Ӱ���������������ʵ������֪�������Ҵ�������������������������������ӡ�

����Ŀ��ijͬѧ�������ϵ�֪25��ʱ��������ĵ��볣�����±�:
�� | HCOOH | HClO | H2CO3 | H2C2O4 | H2S |
���볣��(Ka) | 1.8��10-4 | 3��10-8 | K1=4.4��10-7 K2=4.7��10-11 | K1=5.4��10-2 K2=5.4��10-5 | K1=1.3��10-7 K2=7.1��10-15 |
�ݴ˻ش����¼�������:
(1)��λͬѧ���ݱ�������д�����¼�����Ӧ����ʽ
�ף�![]()
�ң�![]()
����![]()
����![]()
������Ӧ���Գɹ����е���______(��ͬѧ����)��
(2)��ͬѧΪ֤�� HCOOH Ϊ���ᣬ�������·���һ����֤������_______(�����)��
�������²��HCOONa��Һ��pH����7
����HCOOH ��Һ������ʵ�飬���ݺܰ�
��HCOOH��Na2S�ܷ�����Ӧ������������ζ������
������pH�Ʋ�ó����� 0.1 mol/L HCOOH ��Һ��pH=1.37
��HCOONa��H3PO4��Ӧ������ HCOOH
��pH=2��HCOOH��Һϡ����100�����pHԼΪ3.4
(3)��ͬѧȡ10.00 mL 0.1 mol/L H2C2O4����������0.1 mol/L NaOH ��Һ���еζ�����ʹ��������Ũ�ȼ���ǽ���ʵʱ��أ���������ʾ pH=7���ȶ�ʱֹͣ�ζ�����ʱ�������NaOH ��Һ���ΪV mL��
����ʵ���ʹ��________��ȡ10.00 mL 0.1 mol/L H2C2O4(����������)��
��V____10.00 mL(����>����="����<��)��
���ζ���������Һ����������Ũ���ɴ�СΪ��_________(��д����Ũ�ȷ��Ų�����>������)��
����ͬѧ���ͬѧ��ͬ��ʵ�顣������0.1 mol/L H2C2O4����0.1 mol/L H2S��Һ����ͬѧʵ���������Һc(HS-)___ c(S2-)(��">""<"����=��)��
(4)��֪���Ը�����ؿɽ����������ɶ�����̼�������� HCOOH ��Һ��ijKMnO4��Ʒ���д��Ȳⶨ(���ʲ��ε���Ӧ)��ȡ0.200 g KMnO4��Ʒ(M=158 g/mol)����ƿ���ܽⲢ�ữ����0.100 mol/L�ı�HCOOH��Һ���еζ����ζ����յ�ʱ���� HCOOH ��Һ20.00 mL��
���� KMnO
��ȷ���ﵽ�ζ��յ���жϷ�����_________��
������Ʒ��KMnO4�Ĵ���Ϊ _______(�ðٷ�����ʾ)��
����Ŀ��I.��̿�������Ʊ���ʯ������ˮú���ȡ����������գ�
(1)��ʯ����Ҫ�ɷ���CaC2��CaC2�ľ���������___________������ˮ��Ӧ�Ļ�ѧ����ʽΪ______________________��
(2)�Ʊ���ʯ����Ҫ�õ�CaCO3�����CaCO3������Ԫ��ԭ�Ӱ뾶���Ӵ�С��˳������Ϊ____________����ԭ�ӵĺ�������Ų�ʽΪ_________________��
(3)��ͬ����Ԫ��Mg��ȣ�Ca�Ľ����Ը�______������ǿ������������������֤����һ���۵�ʵ����ʵ��________________________��
II.�ý�̿����ˮú���ķ�ӦΪ��C(s)+H2O(g)![]() CO(g)+H2(g)�����������գ�
CO(g)+H2(g)�����������գ�
(4)һ���¶��£���һ���̶��ݻ����ܱ������з���������Ӧ�����в����жϸ÷�Ӧ�ﵽƽ��״̬����____________����ѡ���ţ�
a�������е�ѹǿ���ٸı� b�����������ܶȲ��ٸı�
c��v��(CO)=v��(H2O) d��c(CO)=c(H2)
(5)����ͬ����C (s)��H2O (g)�ֱ���뵽���Ϊ2 L�ĺ����ܱ������У����з�Ӧ���õ��������ݣ�
ʵ���� | �¶�/�� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ���� ��ʱ��/min | ||
H2O | C | H2 | CO | |||
1 | 650 | 0.01 | 0.02 | 0.008 | 5 | |
2 | 800 | 0.02 | 0.03 | 0.017 | 3 | |
��ʵ��1����v(H2) ��ʾ�ĵ���ƽ��ʱ��ƽ����Ӧ����Ϊ____________��
������ͼ����ȷ����________����ѡ���ţ�
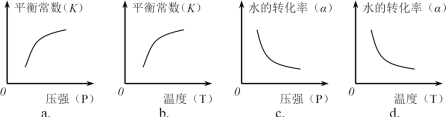
����Ŀ��������ͼʾװ���о����ʵ����ʣ��ܹ���ÿɿ����۵���
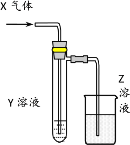
X | Y | Z | ���� | |
A | HCHO | ����Cu(OH)2 | NaOH(aq) | ��֤��ȩ�Ļ�ԭ�� |
B | CH3Cl | AgNO3(aq) | NaOH(aq) | ��֤һ�ȼ��麬��Ԫ�� |
C | SO2 | KMnO4(aq) | NaOH(aq) | ��֤���������Ư���� |
D | Cl2 | ����KI(aq) | NaOH(aq) | ��֤������ǿ������ |
A.AB.BC.CD.D