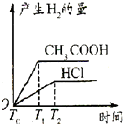
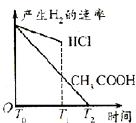
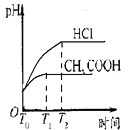
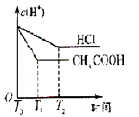
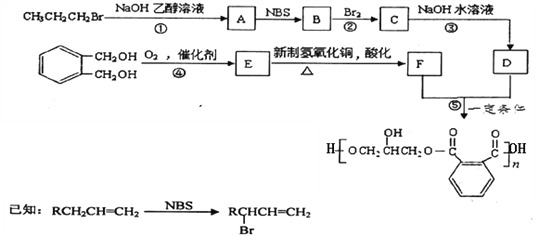
��Ŀ����
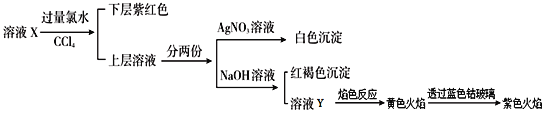
����Ŀ��ij��ҺX�н����ܺ���K+��Na+��Fe2+��Fe3+��SO42-��CO32-��I-��Cl-�е�һ�ֻ���֣�����Һ�и�����Ũ����ͬ���ֽ�������ʵ�飬����˵����ȷ���ǣ� ��
A. ����ҺX��Na+��Cl-һ����
B. ��ҺY�м����Ȼ�����Һû�г���
C. ����Һ�����������������⻯�غ��Ȼ�������ˮ��õ���
D. ԭ��ҺX�еμӼ���KSCN��Һ���ٵμ������Ĵ���������Һ����Һ��Ѫ��ɫ
���𰸡�C
����������ʵ�����̿�֪��������ˮ��Ӧ�����Һ������CCl4��ȡ���²���Ϻ�ɫ��˵��ԭ��Һ�к���I-�����ܹ�����I-��Fe3+����ͬʱ��������Һ�С���ʱ�ϲ��ˮ��Һ��һ��������ˮ��ԭ���ɵ�Cl-�������ø���Һ����AgNO3���ɵİ�ɫ����������Cl-����˵��������ԭ��Һ�С�����һ�ݼ���NaOH�������ɫ����˵����Һ�д���Fe3+�����Ǹ�Fe3+�ֲ��ܴ�����ԭ��Һ�У���ֻ����Fe2+��ʼʱ����ˮ��������Fe3+������ԭ��Һ�к���Fe2+��Fe2+�Ĵ���˵��ԭ��Һ�в�����CO32-��Y����ɫ��ӦΪ��ɫ˵���������ӣ�������ȷ��ԭ��Һ���Ƿ������ӣ����ܲ����۲���ɫ��ӦΪ��ɫ����֤��һ���������ӣ��Դ������
�������Ϸ�����֪I-��Fe2+��K+һ�����ڣ�Fe3+��CO32-һ�������ڣ�SO42-��Cl-��Na+���ܴ��ڣ���
A�����ڹ����м��������Ӻ������ӣ�����ҺX��Na+��Cl-����ȷ���Ƿ��У�A����
B����Һ�п��ܻẬ��������ӣ������Ȼ�����Һ�����ɰ�ɫ������B����
C������Һ��һ������I-��Fe2+��K+�����ܺ�SO42-��Cl-��Na+����Һ�����������������⻯�غ��Ȼ�������ˮ��õ��ģ�C��ȷ��
D�����ڵ����ӵĻ�ԭ��ǿ���������ӣ�ԭ��ҺX�еμӼ���KSCN��Һ���ٵμ������Ĵ���������Һ���������Ӳ�һ������������Һ��һ����Ѫ��ɫ��D����
��ѡC��