��Ŀ����
ijЩ��ѧ��Ӧ������ʽ��ʾ��δ��ƽ����A+B��C+D+H2O
��ش��������⣺
��1����A��C��D��������Ԫ�أ���A����Ԫ�صĻ��ϼ۽���C��D֮�䣬д���÷�Ӧ�����ӷ���ʽ��___________________________________________��
��2����CΪ�Ȼ��ƣ�D����ʹ����ʯ��ˮ����ǵ���ζ���壬��A��B������ǣ�����������A________________��________________ , B______________________��
��3����AΪ�Ϻ�ɫ������DΪ��ɫ�̼������壬��д��������ʽ�Ļ�ѧ����ʽ��____________________________________________��
��4����C��D��Ϊ�����ҷ��Ӿ�����ͬ��ԭ�Ӹ����ȣ��������ʽ��ѧ����ʽ�ǣ�____________________________________________��
��5����AΪ�������ƣ�BΪ���ᣬ��C��____________��D��_______________��
��1��Cl2+2OH��=Cl��+ ClO��+H2O��2�֣�
��2��A��������մ� С�մ� B�����ᣨÿ��1�֣���3�֣�
��3��Cu��2H2SO4��Ũ��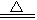 CuSO4��2H2O��SO2����2�֣�
CuSO4��2H2O��SO2����2�֣�
��4��Cʮ2H2SO4��Ũ�� CO2����2SO2����2H2O��2�֣�
CO2����2SO2����2H2O��2�֣�
��5�������ƺ������������������ƣ�д��ѧʽҲ���֣���ÿ��1�֣���2�֣�
���������������1��A����Ԫ�صĻ��ϼ۽���C��D֮�䣬��A��ClԪ�ػ��ϼ۲������ߡ����ֽ��ͣ����ϵķ�ӦΪCl2��ǿ����Һ�ķ�Ӧ�����ӷ���ʽΪ��Cl2+2OH�� ="=" Cl��+ ClO��+H2O��
��2��A��B��Ӧ����NaCl����ζ������CO2��H2O����A����ΪNa2CO3������Ϊ������մ�Ҳ����ΪNaHCO3������ΪС�մ�BΪ���ᡣ
��3��AΪ�Ϻ�ɫ��������ͭ���ʣ�DΪ��ɫ�̼������壬���ϵķ�ӦΪCu��Ũ���ᷴӦ����CuSO4��SO2��H2O���ɵû�ѧ����ʽ��Cu��2H2SO4��Ũ�� CuSO4��2H2O��SO2����
CuSO4��2H2O��SO2����
��4��C��D��Ϊ�����ҷ��Ӿ�����ͬ��ԭ�Ӹ����ȣ����ϵķ�ӦΪC��Ũ���ᷴӦ����CO2��SO2��H2O����ѧ����ʽΪ��Cʮ2H2SO4��Ũ�� CO2����2SO2����2H2O��
CO2����2SO2����2H2O��
��5��Na2O2��H2SO4��Ӧ����Na2SO4��O2��H2O������C��DΪ�����ƺ������������������ơ�
���㣺���⿼�����ӷ���ʽ�뻯ѧ����ʽ����д����Ӧ�����������ƶϡ�

 �±�ΪԪ�����ڱ�ǰ�����ڵ�һ���֣������й�R��W��X��Y��Z����Ԫ�ص���������ȷ����
�±�ΪԪ�����ڱ�ǰ�����ڵ�һ���֣������й�R��W��X��Y��Z����Ԫ�ص���������ȷ����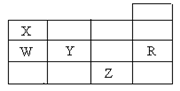
| A����ѹ������Ԫ�صĵ����У�Z���ʵķе���� |
| B��Y��Z�������ӵ��Ӳ�ṹ����Rԭ�ӵ���ͬ |
| C��W���⻯���X���⻯���ȶ� |
| D��YԪ������������Ӧˮ�����WԪ�ص�����������Ӧˮ���������ǿ |
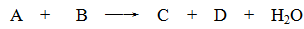
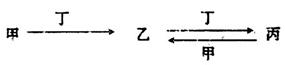
 ��
��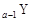 ��
��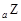 ��
�� ��
��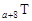 �����ֳ����Ķ�����Ԫ�أ���ԭ�Ӱ뾶��ԭ�������仯����ͼ��ʾ��Z�ǵؿ��к�����ߵ�Ԫ�ء�Wԭ��������������K���������2����
�����ֳ����Ķ�����Ԫ�أ���ԭ�Ӱ뾶��ԭ�������仯����ͼ��ʾ��Z�ǵؿ��к�����ߵ�Ԫ�ء�Wԭ��������������K���������2����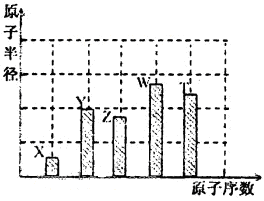
 +18��N��2y+18 B��Z��
+18��N��2y+18 B��Z��