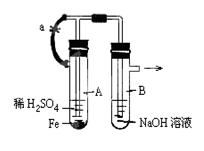
��Ŀ����
[2012��ʯ��ׯ�ʼ�]��12�֣�ij�о���ѧϰС��Ϊ̽��Cu(OH)2���ȷֽ���P�������ʣ��������ʵ�顣
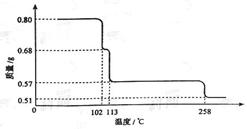
��1��ȡ0.98 g Cu(OH)2������ȣ��������¶ȱ仯��������ͼ1��ʾ������A��B�Ļ�ѧʽ�ֱ�Ϊ �� ��
��2��ȡ��������B����������ϡ���ᣬ�õ���ɫ��Һ��ͬʱ�۲쵽�����л��к�ɫ������ڣ��÷�Ӧ�����ӷ���ʽΪ ��
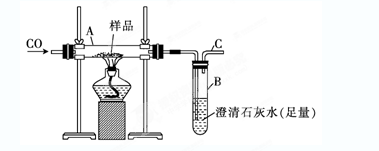
��3��Ϊ֤������A�ڼ���ʱ����NH3��Ӧ��ijͬѧ�������ͼ��(�г�װ��δ����)��ʾʵ��װ�á�
�ټ�������װ�������Եķ��� ��
��ʵ������й۲쵽������������
iֱ�������й����ɺ�ɫ��Ϊ��ɫ��iiװ�����й����ɰ�ɫ��Ϊ��ɫ��
��֤������A��NH3�����˷�Ӧ���ж����ݵ��� (��ѡ����ĸ)��
a��ֻ��i���� b��ֻ��ii���� c��i��ii������

��1��ȡ0.98 g Cu(OH)2������ȣ��������¶ȱ仯��������ͼ1��ʾ������A��B�Ļ�ѧʽ�ֱ�Ϊ �� ��
��2��ȡ��������B����������ϡ���ᣬ�õ���ɫ��Һ��ͬʱ�۲쵽�����л��к�ɫ������ڣ��÷�Ӧ�����ӷ���ʽΪ ��
��3��Ϊ֤������A�ڼ���ʱ����NH3��Ӧ��ijͬѧ�������ͼ��(�г�װ��δ����)��ʾʵ��װ�á�
�ټ�������װ�������Եķ��� ��
��ʵ������й۲쵽������������
iֱ�������й����ɺ�ɫ��Ϊ��ɫ��iiװ�����й����ɰ�ɫ��Ϊ��ɫ��
��֤������A��NH3�����˷�Ӧ���ж����ݵ��� (��ѡ����ĸ)��
a��ֻ��i���� b��ֻ��ii���� c��i��ii������
��12�֣���1��CuO Cu2O
��2��Cu2O��2H+��Cu2+��Cu��H2O
��3����ͼ����װ�ã��رշ�Һ©����������ĩ�˵��ܽ���ˮ�У�����ë����ס��ƿ�ײ��������ܿ�����������ð����������ë������ȴ�����º����γ�һ��Һ���ҳ������䣬��֤��װ������������ b
��2��Cu2O��2H+��Cu2+��Cu��H2O
��3����ͼ����װ�ã��رշ�Һ©����������ĩ�˵��ܽ���ˮ�У�����ë����ס��ƿ�ײ��������ܿ�����������ð����������ë������ȴ�����º����γ�һ��Һ���ҳ������䣬��֤��װ������������ b
��1��������A��CuO��n(CuO) ��0.80g/80g��mol��1��0.01mol����A��Bʧȥ�������ʵ���Ϊ����0.80g��0.72g��/16 g��mol��1��0.005mol��0.72gB�к���0.01molCu��0.005molO��Cu��O�����ʵ���֮��Ϊ2:1������B�Ļ�ѧʽΪCu2O��
��2���÷�Ӧ������Cu2+��Cu���ʸ÷�Ӧ�����ӷ���ʽΪCu2O��2H+��Cu2+��Cu��H2O��
��3������ͼ1֪CuO���ȷֽ����ɺ�ɫ��Cu2O����������i����˵��CuO����ʱ����NH3��Ӧ������ii˵����ˮ���ɣ��Ӷ�˵������ʱCuO����NH3��Ӧ����b��ȷ��
��2���÷�Ӧ������Cu2+��Cu���ʸ÷�Ӧ�����ӷ���ʽΪCu2O��2H+��Cu2+��Cu��H2O��
��3������ͼ1֪CuO���ȷֽ����ɺ�ɫ��Cu2O����������i����˵��CuO����ʱ����NH3��Ӧ������ii˵����ˮ���ɣ��Ӷ�˵������ʱCuO����NH3��Ӧ����b��ȷ��

��ϰ��ϵ�д�
�����Ŀ