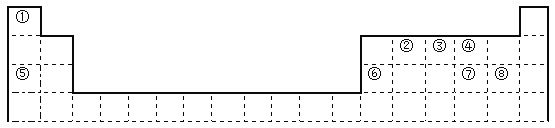
��Ŀ����
����Ŀ�������������(��������)�е��麬���Ƿ꣬ʵ��װ������ͼ��ʾ(�г�װ������ȥ)��

��ʵ��1�����������Һ
��ȡ0.132gAg2O3����NaOH��Һ��ȫ�ܽ�����Ƴ�1LNa3AsO3��Һ������Һ1mL�൱��0.10mg�飩��
��ȡһ����������Һ������1L������Ϊ1mg��L-1�������Һ��
��1��������У�����ʹ�õIJ����������ձ����������⣬����__________���������ȡ�ò������Na3AsO3��Һ____mL��
��ʵ��2���Ʊ��������Һ
����Aƿ�м���2.00mL�����Һ�������μ���һ���������ᡢKI��Һ��SnCl2��Һ�����ȣ����·���10 min��ʹ��Ԫ��ȫ��ת��ΪH3AsO3��
����Aƿ�м�������п��(����ZnS����)����������װ������Ǧ���ĵ�����B����ʹB���Ҳ�ĩ�˲����ɫ��C����������Һ��Һ���£����Ʒ�Ӧ�¶�25~40����45 min�����ɵ��黯�����屻��ȫ���գ�Ag+����ԭΪ��ɫ��̬����
��ȡ��C�ܣ������������ȷ����̶��ߣ����ȣ��õ��������Һ��
��2������Ǧ����������_____________________��
��3����������黯�ⷴӦ�����ӷ���ʽ��
____Zn+____H3AsO3+____H+=____( )+ ____Zn2++____( )________________
��4������A ƿ�з�Ӧ�¶ȵķ�����________________________����Ӧ�У�Aƿ�н϶������������������˽��������⣬�����е�������_____________________________��
��5��B���Ҳ�ĩ�˵��ܿھ����ܹ���(ԼΪ1mm)��ԭ����__________________________��
��ʵ��3���ж���Ʒ���麬���Ƿ�
��ȡag������Ʒ�����ʵ��2�� ���С�2.00m�����Һ�����ظ���ʵ��2��������������ʵ������Һ�����������Һ�ȶԣ�������Һ�����ɫdz��˵������Ʒ������δ���꣬��֮�ꡣ
��6������涨������Ϊ�����֮��(��������)����a��ֵΪ______________________��
���𰸡� 1000mL����ƿ����ͷ�ι� 10.0 ��ȥH2S���� 3Zn+H3AsO3+6H+=AsH3��+3Zn2++3H2O ˮԡ���� ��AsH3������ȫ����C�ܣ������������𰸣� ����Ӧ�Ӵ������ʹAsH3���屻������գ�ȷ���γɽ�̬�� 1.0
����������1��������У�����ʹ�õIJ����������ձ����������⣬����1000mL����ƿ����ͷ�ιܣ�1mL�൱��0.10mg�飬����1L������Ϊ1mg��L-1�������Һ���������ȡ�ò������Na3AsO3��Һ 10.0mL����2��H2S��CH3COO2Pb=PbS����2CH3COOH,����Ǧ���������dz�ȥH2S���壻��3��п��+3�۵��黹ԭ�����黯�⣬�Ȼ�п��ˮ����Ӧ�����ӷ���ʽ3Zn+H3AsO3+6H+=AsH3��+3Zn2++3H2O����4�����Ʒ�Ӧ�¶�25~40�����ɲ���ˮԡ��������Ӧ�У�Aƿ�н϶������������������˽��������⣬����û������װ�ã��ɼ�����һ�����þ��������������е������ǽ�AsH3������ȫ����C�ܣ������������𰸣�����5��B���Ҳ�ĩ�˵��ܿھ����ܹ���(ԼΪ1mm)��ԭ��������Ӧ�Ӵ������ʹAsH3���屻������գ�ȷ���γɽ�̬������6������涨������Ϊ�����֮��(��������)��a��2��10-6=2��10-3L��1��10-6��g��L-1����a=1.0,��a��ֵΪ1.0��