��Ŀ����
����Ŀ�������г����۵�ij��ʳ�þ����ΰ�װ����������˵����
��Ʒ�� | GB5461 |
��Ʒ�ȼ� | һ�� |
�� �� | ʳ�Ρ�����ء������ |
�⺬��(��I�ƣ� | 20~50mg/kg |
��װʱ�� | |
��װ��ҵ |
��1���������⻯�������������·������·�Ӧ����ƽ��ѧ����ʽ������ѧ���������ڿհ״���
____KIO3��___KI��___H2SO4��___K2SO4��___I2��___H2O
��2��������Ӧ���ɵ�I2�������Ȼ�̼���顣�������Ȼ�̼��Һ�м���Na2SO3ϡ��Һ����I2��ԭ���Ի������Ȼ�̼��
��Na2SO3ϡ��Һ��I2��Ӧ�����ӷ���ʽ��_________________________________��
��ijѧ����ƻ������Ȼ�̼�IJ�������Ϊ��
a.��������Ȼ�̼��Һ���ڷ�Һ©���У�
b.��������Na2SO3ϡ��Һ��
c.������²�Һ�塣
�����������©�IJ����������������е�λ����______________________��
��3����֪��I2��2S2O32����2I����S4O62����ijѧ���ⶨʳ�þ����εĵ⺬�����䲽��Ϊ��
a. ȷ��ȡwgʳ�Σ�����������ˮʹ����ȫ�ܽ⣻
b.��ϡ�����ữ������Һ����������KI��Һ��ʹKIO3��KI��Ӧ��ȫ��
c.�Ե���Ϊָʾ������μ������ʵ���Ũ��Ϊ2.0��10��3mol��L��1��Na2S2O3��Һ10.0mL��ǡ�÷�Ӧ��ȫ��
���ж�c�з�Ӧǡ����ȫ���ݵ�������______________________��
��b�з�Ӧ��������I2�����ʵ�����___________mol��
����������ʵ��Ͱ�װ��˵�������⾫���εĵ⺬���ǣ��Ժ�w�Ĵ���ʽ��ʾ��
_______________________mg/kg��
���𰸡�1 5 3 3 3 3 I2��SO32����H2O![]() 2I����SO42����2H�� �ڲ���b�����Ӳ���������Һ©��������� ��Һ����ɫǡ�ñ�Ϊ��ɫ 1.0��10��5 4.2 ��102/w
2I����SO42����2H�� �ڲ���b�����Ӳ���������Һ©��������� ��Һ����ɫǡ�ñ�Ϊ��ɫ 1.0��10��5 4.2 ��102/w
��������
��1���÷�Ӧ�У�KIO3��IԪ�ػ��ϼ���+5�۱�Ϊ0�ۡ�KI��IԪ�ػ��ϼ���-1�۱�Ϊ0�ۣ�����ת�Ƶ��������ƽ����ʽ��
��2����I2���������ԣ��ܽ�SO32-����ΪSO42-����������ԭΪI-��
������������߷�Ӧ����֣�
��3���ٶ���ǡ�÷�Ӧʱ����Һ����ɫǡ�ñ�Ϊ��ɫ��
�ڸ���I2+2S2O32-=2I-+S4O62-�е�����������֮��Ĺ�ϵʽ���㵫�����ʵ�����
�۸��ݵ��ʳ������֮�Ƚ��м��㡣
��1���÷�Ӧ�У�KIO3��IԪ�ػ��ϼ���+5�۱�Ϊ0�ۡ�KI��IԪ�ػ��ϼ���-1�۱�Ϊ0�ۣ�ת�Ƶ�������Ϊ5���ٽ��ԭ���غ���ƽ����ʽΪKIO3+5KI+3H2SO4=3K2SO4+3I2+3H2O���ʴ�Ϊ��1��5��3��3��3��3��
��2����I2���������ԣ��ܽ�SO32-����ΪSO42-����������ԭΪI-�����ӷ���ʽΪI2+SO32-+H2O=2I-+SO42-+2H+���ʴ�Ϊ��I2+SO32-+H2O=2I-+SO42-+2H+��
������������߷�Ӧ����֣������ڲ���b�����Ӳ���������Һ©��������ã��ʴ�Ϊ���ڲ���b�����Ӳ���������Һ©��������ã�
��3���ٵ���������Һ����ɫ���������ȫ��Ӧ������Һ������ɫת��Ϊ��ɫ�����Ե���Һ����ɫת��Ϊ��ɫʱ˵����Ӧ��ȫ���ʴ�Ϊ����Һ����ɫǡ�ñ�Ϊ��ɫ��
���������ʵ���Ϊx��
I2+2S2O32-=2I-+S4O62-
1mol 2mol
x 1.00��10-3molL-1��0.024L
1mol��2mol=x����1.00��10-3molL-1��0.024L��
x=![]()
=1.2��10-5mol��
�ʴ�Ϊ��1.2��10-5��
�۸���KIO3+5KI+3H2SO4=3K2SO4+3I2+3H2O��![]() n��I2��=��KIO3��=4��10-6mol��������е������=4��10-6mol��127g/mol=0.508mg��
n��I2��=��KIO3��=4��10-6mol�������������=4��10-6mol��127g/mol=0.508mg��
��ÿkgʳ���е������Ϊy��
��y��1000g=0.508mg��wg��
y=![]() =
=![]() mg��
mg��![]() mg��
mg��

����Ŀ���о�����CO2�ŷ���һ����Ҫ���⡣CO2��������������ɵ�̼�л����Ҫ�����·�Ӧ��
��Ӧ����CO2(g)��3H2(g) ![]() CH3OH(g)��H2O(g) ��H1����49.6 kJ/mol
CH3OH(g)��H2O(g) ��H1����49.6 kJ/mol
��Ӧ����CH3OCH3(g)��H2O(g) ![]() 2CH3OH(g) ��H2����23.4 kJ/mol
2CH3OH(g) ��H2����23.4 kJ/mol
��Ӧ����2CO2(g)��6H2(g) ![]() CH3OCH3(g)��3H2O(g) ��H3
CH3OCH3(g)��3H2O(g) ��H3
��1����H3��________kJ/mol��
��2�����º��������£����ܱ�������ͨ������ʵ�����CO2��H2��������ӦI������������˵����ӦI�ﵽƽ��״̬����_______������ţ���
A����Ӧ��ϵ��ѹǿ���ֲ���
B�������ڵĻ��������ܶȱ��ֲ���
C��ˮ�����ж���2NA��H-O����ͬʱ������ж���3NA��H-H��
D��CH3OH��H2O��Ũ��֮�ȱ��ֲ���
��3����ӦII��ij�¶��µ�ƽ�ⳣ��Ϊ0.25�����¶��£����ܱ������м�������ʵ�����CH3OCH3(g)��H2O(g)����Ӧ��ijʱ�̲�ø����Ũ�����£�
���� | CH3OCH3(g) | H2O(g) | CH3OH(g) |
Ũ��/mol��L��1 | 1.8 | 1.8 | 0.4 |
��ʱv��___v������������������������������������Ӧ�ﵽƽ��״̬ʱ�����������CH3OH�������(CH3OH)% ��___%��
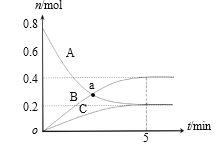
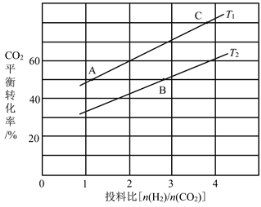
��4����ijѹǿ�£���ӦIII�ڲ�ͬ�¶ȡ���ͬͶ�ϱ�ʱ��CO2��ƽ��ת������ͼ��ʾ��T1�¶��£���6mol CO2��12mol H2����2 L���ܱ������У�5min��Ӧ�ﵽƽ��״̬����0��5min�ڵ�ƽ����Ӧ����v(CH3OCH3)��____��KA��KB��KC����֮��Ĵ�С��ϵΪ____��
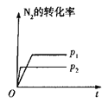
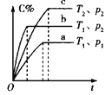
��5����ѹ�½�CO2��H2�������1��3��ϣ��ڲ�ͬ���������·�����ӦI�ͷ�ӦIII������ͬ��ʱ�����CH3OH��ѡ���ԺͲ������¶ȵı仯��ͼ�����У�CH3OH��ѡ���ԣ�![]() ��100%
��100%

�����������ºϳɼ״��Ĺ�ҵ������____��
A��210�� B��230�� C������CZT D������CZ(Zr��1)T