��Ŀ����
11��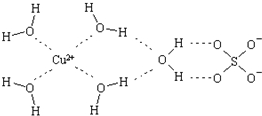 ��ѧ��ͨ��X�����Ʋ���мȺ�����λ�����ֺ����������ṹʾ��ͼ�ɼ�ʾ���£�������λ����������������߱�ʾ��
��ѧ��ͨ��X�����Ʋ���мȺ�����λ�����ֺ����������ṹʾ��ͼ�ɼ�ʾ���£�������λ����������������߱�ʾ����1��д����̬Cuԭ�ӵĵ����Ų�ʽ1s22s22p63s23p63d104s1��[Ar]3d104s1��
��2��Cu2+������NH3��Cl-���γ���λ��Ϊ4������
��[Cu��NH3��4]2+�д��ڵĻ�ѧ��������AC������ţ���
A����λ�� B�����Ӽ� C�����Թ��ۼ� D���Ǽ��Թ��ۼ�
����֪[Cu��NH3��4]2+���жԳƵĿռ乹�ͣ�[Cu��NH3��4]2+�е�����NH3������
Cl-ȡ�����ܵõ����ֲ�ͬ�ṹ�IJ����[Cu��NH3��4]2+�Ŀռ乹��Ϊƽ�������Σ�
��3��ʵ��֤�����������ܶȷ���õ�H2O����Է����������û�ѧʽ�����������Է�������Ҫ����ԭ�����ڽӽ�ˮ�е��ˮ�����д���һ��������ˮ���������������ϡ��γɵĵϷ��ӵ���Է���������ˮ������
��4��������ͭˮ��Һ����μ��백ˮֱ����������Ӧ�����ӷ���ʽΪCu2++2NH3•H2O=Cu��OH��2��+2NH4+��Cu��OH��2+4NH3=Cu��NH3��42++2OH-��
���� ��1��ͭ��29��Ԫ�أ���ԭ�Ӻ�����29�����ӣ����ݹ���ԭ����д���̬ԭ�Ӻ�������Ų�ʽ��
��2����������[Cu��NH3��4]2+�к�����λ������ͬ�ǽ���Ԫ��֮���γɼ��Թ��ۼ���
���γ�4����λ�������жԳƵĿռ乹�ͣ�����Ϊƽ�������λ��������壬��Ϊ�������壬[Cu��NH3��4]2+�е�����NH3������Cl-ȡ����ֻ��һ�ֽṹ��
��3����Ϊˮ���Ӽ��������������ʹˮ���ӳ�Ϊ��ˮ���ӣ�ʹ�������С�������������ܶȷ���õ�H2O����Է������������ۼ����������Է������ϴ�
��4����ˮ������ͭ��Ӧ����������ͭ��ɫ����������ˮ����ʱ����ˮ��������ͭ��Ӧ���ɿ����Ե�ͭ������
��� �⣺��1��ͭ��29��Ԫ�أ���ԭ�Ӻ�����29�����ӣ����ݹ���ԭ��֪�����̬ԭ�Ӻ�������Ų�ʽΪ��1s22s22p63s23p63d104s1��[Ar]3d104s1��
�ʴ�Ϊ��1s22s22p63s23p63d104s1��[Ar]3d104s1��
��2����[Cu��NH3��4]2+��Cu2+��NH3֮��Ļ�ѧ��Ϊ��λ����N-HΪ���Թ��ۼ����ʴ�Ϊ��AC��
���γ�4����λ�������жԳƵĿռ乹�ͣ�����Ϊƽ�������λ��������壬��Ϊ�������壬[Cu��NH3��4]2+�е�����NH3������Cl-ȡ����ֻ��һ�ֽṹ������ӦΪƽ�������Σ��ʴ�Ϊ��ƽ�������Σ�
��3����Ϊˮ���Ӽ��������������ʹˮ���ӳ�Ϊ��ˮ���ӣ�ʹ�������С�������������ܶȷ���õ�H2O����Է������������ۼ����������Է������ϴ�
�ʴ�Ϊ���ڽӽ�ˮ�е��ˮ�����д���һ��������ˮ���������������ϡ��γɵĵϷ��ӵ���Է���������ˮ��������
��4����ˮ������ͭ��Ӧ����������ͭ��ɫ����������ˮ����ʱ����ˮ��������ͭ��Ӧ���ɿ����Ե�ͭ�������������ܽ�õ�����ɫ������Һ���йط�Ӧ���ӷ���ʽΪ��Cu2++2NH3•H2O=Cu��OH��2��+2NH4+��Cu��OH��2+4NH3=Cu��NH3��42++2OH-��
�ʴ�Ϊ��Cu2++2NH3•H2O=Cu��OH��2��+2NH4+��Cu��OH��2+4NH3=Cu��NH3��42++2OH-��
���� ���⿼������ݽ��ӣ��漰������Ԫ�غ�������Ų�����������ṹ�������Ľṹ�й�֪ʶ���ѶȲ���ע�ضԻ���֪ʶ�Ŀ��飮

| A�� | �ߴ��ȵĹ赥�ʹ㷺�����������ά�����ά��ǿ��ᡰ��·�� | |
| B�� | ���Ĺ̶�ֻ���ڸ��¡���ѹ�������������²���ʵ�� | |
| C�� | ���ø����������ҩ�ý��һ�����彡���������Σ�� | |
| D�� | ������أ�K2FeO4����һ�����͡���Ч�����ˮ������������ɱ���������ܾ�ˮ |
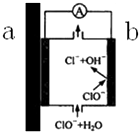 ����ˮ�ܵ������˴������ܡ���п�ܡ�PVC�ܡ�PPR���۹ܵȽΣ������ܡ���п�ܱ����õ�ԭ��֮һ��������ԭ���ԭ�������ͣ�ʾ��ͼ��ͼ��ʾ�������й�˵������ȷ���ǣ�������
����ˮ�ܵ������˴������ܡ���п�ܡ�PVC�ܡ�PPR���۹ܵȽΣ������ܡ���п�ܱ����õ�ԭ��֮һ��������ԭ���ԭ�������ͣ�ʾ��ͼ��ͼ��ʾ�������й�˵������ȷ���ǣ�������| A�� | ����Ƕ�п�ܣ���a��ΪZn���Ǹ���������Zn2+����������ɹܵ���ʴ��Zn2+��������ˮҲ�������к� | |
| B�� | b�˷����ĵ缫��ӦΪ��ClO-+H2O-2e-�TCl-+2OH- | |
| C�� | ���ڸ�ԭ���ԭ���Ĵ��ڣ�һ���̶��ϼ���������ˮ�����ȵ�ɱ���������� | |
| D�� | ������ˮ�����û�����������ܵ��ij��ڽӴ�������ˮ������Է����˱仯 |
| A�� | �ﵽƽ��������Ӧ��ֹͣ������Ӧ���ʵ����淴Ӧ������Ϊ0 | |
| B�� | ���տ�����0.2 mol NH3 | |
| C�� | ���{�¶ȣ���Ӧ�������� | |
| D�� | ��V����N2����V ����H2��=1��3ʱ��һ���ﻯѧƽ��״̬ |
| A�� | pH=2��HA��Һ��pH=12��MOH��Һ����Ȼ�ϣ�c��H+��+c��M+���Tc��OH-��+c��A-�� | |
| B�� | ���ʵ���Ũ����ȵ�CH3COONa��NaOH��Na2CO3������Һ��pH��NaOH����pH��CH3COONa����pH��Na2CO3�� | |
| C�� | NaHCO3��Һ�У�c��OH-��-c��H+��=c��H2CO3��-c��CO32-�� | |
| D�� | 10��ʱpH=12��NaOH��Һ��40��ʱpH=12��NaOH��Һ��c��OH-����� |
2SO2��g��+O2��g��$?_{��}^{����}$2SO3��g����H=-92.3KJ/mol
��Ӧ�����У�SO2��O2��SO3�����ʵ�����mol���ı仯���±���0��4minʱ����������ѹǿΪ101KPa����
| ʱ��min | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
| n��SO2�� | 2.00 | 1.92 | 1.84 | 1.76 | 1.76 | 1.64 | 1.52 | 1.40 | 1.40 | 1.40 |
| n��O2�� | 1.00 | 0.96 | 0.92 | 0.88 | 0.88 | 0.82 | 0.76 | 0.70 | 0.70 | 0.70 |
| n��SO3�� | 0 | 0.08 | 0.16 | 0.24 | 0.24 | 0.36 | 0.48 | 0.60 | 0.60 | 0.60 |
��3��4min��7��9minʱ�Σ���Ӧ����ƽ��״̬��
�ڵ�5minʱ����ͬʱ������ʺ�ת������������������ı���������������ѹǿ��ƽ�����������ƶ���

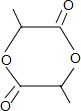 +2H2O��
+2H2O��