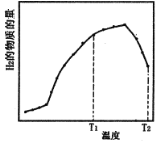
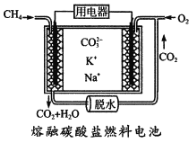
��Ŀ����
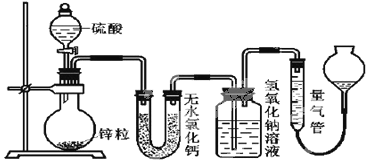
����Ŀ����������������Ԫ�أ��ڷβ���Ѫ�쵰����Ѫ���ص�Fe2����O2��ϣ���O2�͵�������֯���١������벻���������ȱ����ƶѪ����ľ���к��бȽϷḻ����Ԫ�أ�ij��ѧС��ⶨ�京����
��1������Ԫ�صķ��룩 ������ľ������֮ϴ�����飬������ˮ���ݣ�������Һ��δ�ܼ���Ԫ�ء��������и������պ�ľ����ʹ֮��ȫ�һ��������ֽ����ܽ⣬���ˣ���Һ���á�
�� ����Һ��ⲻ����Ԫ�ص�ԭ����____��
�� ��Һ����Ԫ�صĴ�����ʽ��Fe2����____��
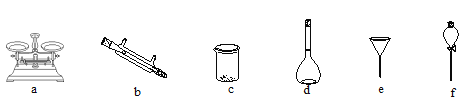
��2����������Һ�� ����Һ���Ƴ�100 mL ��Һ��ʵ��ʱ��Ҫѡ����ʵ�������ɣ���Ԫ�صķ��룩����������Һ��2��ʵ�飬������������ʹ�õ���____��
��3������Ԫ�غ����ⶨ�� ��ѧС��������²���������
��. ������������Һ�м�������NaOH��Һ������ʹFe��OH��2ȫ��ת��ΪFe��OH��3Ȼ����ˡ�ϴ�ӳ��������Ⱥ�ɡ�����Fe��OH��3���������з�������ָ���ò��������Ƿ���м�������____��
��. ��ɫ��������ʾ�����£�
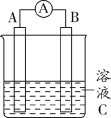
![]()
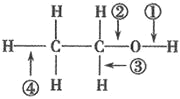
�� �����ӷ���ʽ���ͼ���H2O2��Ŀ����____��
�� ��Һa����ɫ��____��
�� ��Һ��ɫԽ����������Խ�������ԽС��������H2O2�������ľ������Ԫ�صĺ���____���ƫ��ƫС������
��4���������ױ���������������Fe2������O2������ȴ������������������һ�ּ�����ˮ���֡�������ˮ���ֵĴ���ӣ�ΪѪ�����ṩ����ˮ�������ɼ���������ԭ��Ӧ�ܷ�����___�йء�
���𰸡���ľ���к���Ԫ�ص����ʲ�����ˮ Fe3�� b��f �����У� Fe��OH��3�����ֽ⣬�ҳ������������������ H2O2 �� 2H�� ��2Fe2�� ��2Fe3�� ��2H2O ��ɫ ƫС ˮ
��������
(1)�ٽ���Һ��ⲻ����Ԫ��˵����Ԫ�ش��ڵ�����û������ˮ���ڸ��ݸ������պ�ľ��������������Ӳ��ȶ������жϣ�
(2)ͨ������ʵ������Ԫ�صķ���������������Һ��2��ʵ�飬���2��ʵ����̷�����Ҫ���������н��
(3)��Fe(OH)3�����ֽ⣻�ٹ��������ܹ�������������Ϊ�����ӣ�������������KSCN��Һ�γ�Ѫ��ɫ��Һ���۸��ݲⶨԭ�����������ɫ�����жϣ�
(4)���ݵ�������һ�ּ�����ˮ���֡�������ˮ���ֵĴ���ӣ�ΪѪ�����ṩ����ˮ���������жϡ�
(1)�ٽ���Һ��ⲻ����Ԫ�أ���������Ϊ��ľ���к���Ԫ�ص����ʲ�����ˮ���ʴ�Ϊ����ľ���к���Ԫ�ص����ʲ�����ˮ��
���������и������պ�ľ����ʹ֮��ȫ�һ�����ľ���е���Ԫ�ػ�ת��Ϊ�����������Ϊ�������Ӳ��ȶ�����˵õ���������һ���������������������ܽ⣬���ˣ���Һ����Ԫ�صĴ�����ʽ��Fe2+��Fe3+���ʴ�Ϊ��Fe3+��
(2)����Һ���Ƴ�100mL��Һ��Ȼ���������Ԫ�صķ���������������Һ��2��ʵ�飻������Ԫ��ͨ�����˳�ȥ�������Ҫ©�������������ձ��ȣ�������Һ��Ҫ�������ܽ⡢ת�ơ�ϴ�Ӷ��ݵȣ���Ҫ����������ƽ���ձ�������ƿ������������ͷ�ιܵȣ���ͼ������һ�����õ��������ܡ���Һ©������ѡbf���ʴ�Ϊ��bf��
(3)������Һ�м�������NaOH��Һ������ʹFe(OH)2ȫ��ת��ΪFe(OH)3Ȼ����ˡ�ϴ�ӳ��������Ⱥ�ɡ�����Fe(OH)3���������ò�����Fe(OH)3�����ֽ⣬�õ���Fe(OH)3������һ�����ڽϴ�������������������࣬�����������ʵ��ȷ�ȵͣ���˸ò������������У��ʴ�Ϊ�������У�Fe(OH)3�����ֽ⣬�ҳ������������������
�ټ���H2O2��Ŀ���������������ӣ���Ӧ�����ӷ���ʽΪ��H2O2+2H++2Fe2+=2Fe3++2H2O���ʴ�Ϊ��H2O2+2H++2Fe2+=2Fe3++2H2O��
�������ӽ��SCN-��������Fe(SCN)3����Һ��Ѫ��ɫ���ʴ�Ϊ����ɫ(��Ѫ��ɫ)��
����Һ��ɫԽ����������Խ�������ԽС��������H2O2�������������ʴ��²����ľ������Ԫ�صĺ���ƫС���ʴ�Ϊ��ƫС��
(4)��������һ�ּ�����ˮ���֡�������ˮ���ֵĴ���ӣ�ΪѪ�����ṩ����ˮ�������������ױ���������������Fe2+����O2������ȴ����������˵��������ԭ��Ӧ�ܷ�����ˮ�йأ��ʴ�Ϊ��ˮ��

����Ŀ����Ԫ�صĵ��ʺͳ����Ļ������ڹ���ũҵ��������;�㷺��
��1����ҵ�����÷�������ķ����õ���������������Ҫ�ɷֵķе����£�
N2 | O2 | Ar | CO2 |
-196�� | -183�� | -186�� | -78�� |
�ֽ����������ȴҺ����Ȼ�������£������ȷ��������������______��
��2������ʱ�����е�N2ת��ΪNO��������NO��______ɫ�����壬______������������������������ˮ��NO�ڿ����к����ױ�������NO2��NO2����ˮ������ѧ��Ӧ��д��NO2��ˮ��Ӧ�Ļ�ѧ����ʽΪ__________________��
��3��ʵ���ҿ��ù���NH4Cl�����Ca��OH��2���ȷ�Ӧ��ȡ������
����ȡ�����Ļ�ѧ����ʽΪ__________________��
��Ҫ��ȡ��״����4.48L�İ�����������Ҫ��ȡ����NH4Cl������Ϊ______g��
��4����֪��4NH3+6NO![]() 5N2+6H2O��ѧ�о���ѧϰС���ͬѧ�ڼ�����Ա��ָ���£����������̣�̽����ͬ������NH3��ԭNO��Ӧ�Ĵ����ܣ�
5N2+6H2O��ѧ�о���ѧϰС���ͬѧ�ڼ�����Ա��ָ���£����������̣�̽����ͬ������NH3��ԭNO��Ӧ�Ĵ����ܣ� ![]()
����������ʵ����������ͬ���ڴ���Ӧ����װ�ز�ͬ�Ĵ�������������Ӧ��Ļ�����壬ͨ��һ��������з�̪��ϡ������Һ����Һ�������Ũ�Ⱦ���ͬ����
��NH3��ϡ������Һ��Ӧ�����ӷ���ʽΪ__________________��
��Ϊ�˱Ƚϲ�ͬ�����Ĵ����ܣ���Ҫ��������¼��������____________��