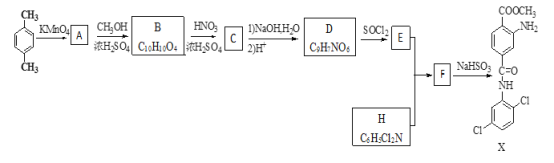
��Ŀ����
����Ŀ���������ӷ���ʽ��ȷ����
A.�Ȼ�þ��Һ�백ˮ��Ӧ��Mg2+��2OH-��Mg(OH)2��
B.Ba(OH)2��H2SO4��Һ ��ϣ�Ba2++ SO42�� == Ba SO4��
C.�� NH4HCO3 ��Һ�мӹ����� NaOH ��Һ�����ȣ�NH4��OH��===NH3����H2O
D.���������Һ��ͨ�������CO2��SiO32��+2CO2 + 2H2O == H2SiO3��+2HCO3��
���𰸡�D
��������
A��NH3��H2OΪ���Ӧ���û�ѧʽ��ʾ���Ȼ�þ��Һ�백ˮ��Ӧ�����ӷ�ӦΪMg2++2NH3��H2O�TMg(OH)2��+2NH4+����A����
B��Ba(OH)2��H2SO4��Һ��Ϸ�Ӧ���ɳ�����ˮ��H2SO4��Ba(OH)2��Һ��Ӧ�����ӷ�ӦΪBa2++2OH-+2H++SO42-�TBaSO4��+2H2O����B����
C����NH4HCO3��Һ�мӹ�����NaOH��Һ�����ȣ�̼���������ҲҪ�����������ӷ�Ӧ�����ӷ���ʽ��NH4++HCO3-+2OH- ![]() NH3��+2H2O+CO32-����C����
NH3��+2H2O+CO32-����C����
D����������Һ��ͨ�����CO2�����ӷ���ʽ��SiO32-+2CO2+2H2O�TH2SiO3��+2HCO3-����D��ȷ��
��ѡD��

����Ŀ��ij��ѧ��ȤС�����I2-CCl4����ȡ�Ⲣ����CCl4���������ϵõ�������Ϣ��
I2 | �Ϻ�ɫ���壬�е�184.3��C��������������������������ˮ�е��ܽ�Ⱥ�С�������������л��ܼ��� |
CCl4 | ��ɫ�ж�Һ�壬�е�76.8�棬�ӷ�����ˮ�������ܣ������Ҵ������ѡ��ȷ¼�ʯ���ѵȻ��ܣ�����ȼ�գ������л��ܼ��� |
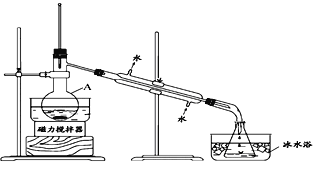
I����ȤС����ݱ�����Ϣ�������ѧ֪ʶ������ͼ��װ��������ʵ�飺
��1����װ��������A��������____________��
��2����ƿ���ڱ�ˮ�е�Ŀ����_____________��
��3��ʵ�������ֵõ���CCl4��dz�Ϻ�ɫ��Ϊ��һ���ᴿCCl4������ó��ӷ�Һ�ķ��������ᴿ�������в���������ȷ���ǣ�_______________________________��
�پ��ã���Һ��ֲ��Һ �ڼ����Թ�����Na2SO3��Һ �۳�������� �ܽ���������Һת�Ƶ���Һ©���� �ݶԷ�Һ©�����м�©
II�����ڷ���Iû�дﵽԤ��Ŀ�꣬��ȤС�����趨���·�������ʵ�飺
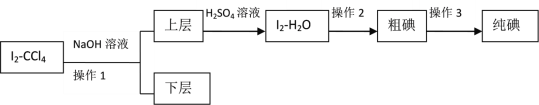
��֪��3I2��6NaOH��5NaI��NaIO3��3H2O
��4������2��������____________��
��5���μ���������ʱ����Һ��ɫ��ƣ���д����Ӧ�����ӷ���ʽ��________________��
��6����ʵ���б���Ҫ����NaOH��Һ��Ũ�Ƚ�_______���������������������������_______��������������С�����������ǣ�__________