��Ŀ����
1��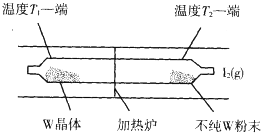 ���á���ѧ����ת�Ʒ����ᴿ�����ٵķ�Ӧԭ��Ϊ��W��s��+I2��g��?WI2��g����H��0
���á���ѧ����ת�Ʒ����ᴿ�����ٵķ�Ӧԭ��Ϊ��W��s��+I2��g��?WI2��g����H��0��1���÷�Ӧ��ƽ�ⳣ������ʽK=$\frac{c��W{I}_{2}��}{c��{I}_{2}��}$��
��2���ں��º��������£��ܹ�˵��������Ӧһ���ﵽƽ��״̬����bc������ţ���
a��I2��g����WI2��g����Ũ�����
b��W���������ٱ仯
c�������ڻ��������ܶȱ��ֲ���
d�������ڻ�������ѹǿ���ֲ���
��3����һ���¶��£���ƽ�ⳣ��K=$\frac{1}{3}$����2L�����ܱ������м���l mol I2��g��������W��s����5min��Ӧ�ﵽƽ�⣮��0-5min�ڵ�ƽ����Ӧ����v��WI2��=0.025mol/��L•min����ƽ��ʱI2��g����ת����Ϊ25%����ͬ�¶��£�����ʼ����I2��g�������ʵ�����ԭ����2������������ֵ��ԭ����2������c������ţ���
a��ƽ�ⳣ�� b���ﵽƽ���ʱ��c��I2�����ʵ���Ũ�� �� d��ƽ��ʱWI2���������
��4��Ϊ��߸÷�Ӧ�ķ�Ӧ���ʣ�ͬʱʹ$\frac{c��{I}_{2}��}{c��W{I}_{2}��}$���ɲ�ȡ�Ĵ�ʩΪ�����¶ȣ�
��5����ҵ������������Ӧԭ���ᴿ�����ٵ�ʾ��ͼ���£�
��Ӧ��ʯӢ��չ��н��У������¶�ΪT2��һ�˷���δ�ᴿ��W��ĩ������I2��g����һ��ʱ������¶�ΪT1��һ�˵õ��˴����ľ��壬���¶�T1��T2���������������=������������Ӧ��ϵ��ѭ��ʹ�õ�������I2��
���� ��1��ƽ�ⳣ��ָһ�������£����淴Ӧ�ﵽƽ��ʱ��������Ũ�Ȼ�ѧ������������֮���뷴Ӧ��Ũ�Ȼ�ѧ������������֮���ı�ֵ��ע����塢��Һ�岻��Ҫд����
��2������Ӧ�ﵽƽ��״̬ʱ�����淴Ӧ������ȣ������ʵ�Ũ�Ȳ��ٸı䣬�ɴ�������һЩ������Ҳ���䣬�Դ˽����жϣ�ע��ѡ����������淴Ӧ���з����仯�����������ɱ仯�����仯˵������ƽ�⣻
��3���跴Ӧ�ĵⵥ�����ʵ���Ϊx
W��s��+I2��g��?WI2��g��
��ʼ����mol�� 1 0
�仯����mol�� x x
ƽ������mol�� 1-x x
K=$\frac{x}{1-x}$=$\frac{1}{3}$
x=0.25mol
��0-5min�ڵ�ƽ����Ӧ����v��WI2��=$\frac{��c}{��t}$��ת����=$\frac{������}{��ʼ��}$��100%��
��Ӧǰ���������ʵ������䣬����ⵥ�����ʵ���Ϊԭ����2����I2�����ʵ���Ũ��Ϊԭ����2����ƽ�ⳣ�����¶ȱ仯��Ũ������ﵽƽ���ʱ��̣����ﵽ��ƽ����ͬ��
��4��Ϊ��߸÷�Ӧ�ķ�Ӧ���ʣ�ͬʱʹ$\frac{c��{I}_{2}��}{c��W{I}_{2}��}$����˵��ƽ��������У���Ӧ�Ƿ��ȷ�Ӧ����������Ӧ���ʣ�ƽ��������У�
��5����ͼ��֪���ڸ��������µõ�����W���壬�������¶�ƽ�����淴Ӧ�����ƶ�����Ӧ��ϵ��I2��ѭ��ʹ�ã�
��� �⣺��1���ٿ��淴ӦW��s��+I2��g��?WI2��g����ƽ�ⳣ��k=$\frac{c��W{I}_{2}��}{c��{I}_{2}��}$���ʴ�Ϊ��$\frac{c��W{I}_{2}��}{c��{I}_{2}��}$��
��2��a��ƽ��ʱI2��WI2Ũ�ȹ�ϵ��ת�����йأ�����Ũ�Ȳ�һ����ȣ���a����
b���淴Ӧ���У�W���������仯����W���������ٱ仯��˵������ƽ�⣬��b��ȷ��
c���淴Ӧ���У������������������仯�������������ܶ�Ҳ�ڲ��ϱ仯���������ڻ��������ܶȱ��ֲ��䣬˵����Ӧ�ﵽƽ�⣬��c��ȷ��
d����λʱ���ڣ����������ĵ����ʵ�����⻯�����ɵ����ʵ�����ȣ���Ӧ������ѹǿʼ�ղ��䣬����˵���ﵽƽ�⣬��d����
�ʴ�Ϊ��bc��
��3���跴Ӧ�ĵⵥ�����ʵ���Ϊx
W��s��+I2��g��?WI2��g��
��ʼ����mol�� 1 0
�仯����mol�� x x
ƽ������mol�� 1-x x
K=$\frac{x}{1-x}$=$\frac{1}{3}$
x=0.25mol
��0-5min�ڵ�ƽ����Ӧ����v��WI2��=$\frac{��c}{��t}$=$\frac{\frac{0.25mol}{2L}}{5min}$=0.025mol/��L•min����
ƽ��ʱI2��g����ת����=$\frac{������}{��ʼ��}$��100%=$\frac{0.25mol}{1mol}$��100%=25%��
��Ӧǰ���������ʵ������䣬����ⵥ�����ʵ���Ϊԭ����2����I2�����ʵ���Ũ��Ϊԭ����2����ƽ�ⳣ�����¶ȱ仯��Ũ������ﵽƽ���ʱ��̣����ﵽ��ƽ����ͬ����ѡc��
�ʴ�Ϊ��0.025mol/��L•min����25%��c��
��4��Ϊ��߸÷�Ӧ�ķ�Ӧ���ʣ�ͬʱʹ$\frac{c��{I}_{2}��}{c��W{I}_{2}��}$����˵��ƽ��������У���Ӧ�Ƿ��ȷ�Ӧ����������Ӧ���ʣ�ƽ��������У��ʴ�Ϊ�������¶ȣ�
��5����ͼ��֪���ڸ��������µõ�����W���壬�������¶�ƽ�����淴Ӧ�����ƶ�������ӦΪ���ȷ�Ӧ������H��0��ͨ�������¶�T2�����ڷ�Ӧ������У�Ϊ���£��¶�T1�����ڷ�Ӧ������У�Ϊ���£�����T1��T2����Ӧ��ϵ��I2��ѭ��ʹ�ã�
�ʴ�Ϊ������I2��
���� ���⿼�黯ѧƽ�ⳣ������ѧƽ����㡢������ʵĵ���ƽ�ⳣ������Һ����Ũ�ȴ�С�Ƚϣ����ؿ���ѧ����֪ʶǨ������������ע��Ƚ���Һ�и�������Ũ����Դ�СʱҪ��ϵ���غ�������غ�ȷ�������Ѷ��еȣ�

 ��ǰ����ϵ�д�
��ǰ����ϵ�д�| A�� | SO2 | B�� | Cl2 | C�� | NO2 | D�� | N2 |
| A�� | ƽ��������Ӧ�����ƶ� | B�� | ����B��ת���ʽ��� | ||
| C�� | x+y��z | D�� | ����C������������� |
| A�� | X������һ������M | |
| B�� | Y������һ������N | |
| C�� | X��Y��������һ������M��N�������� | |
| D�� | ���Ƿ��ȷ�Ӧ���ʲ��ؼ��ȷ�Ӧ��һ���ܷ��� |
| A�� | ������ˮ��ʵ���ȡˮ��Һ������AgNO3��Һ������±������ | |
| B�� | ������ˮ��ʵ���ȡˮ��Һ������KMnO4��H+����Һ����������Ҵ� | |
| C�� | ��������ȥʵ������嵼��KMnO4��H+����Һ�����������ϩ | |
| D�� | ��������ȥʵ������嵼��Br2��CCl4����Һ�����������ϩ |
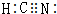 ��HCN��CԪ�صĻ��ϼ�Ϊ+2�ۣ�
��HCN��CԪ�صĻ��ϼ�Ϊ+2�ۣ� ��
��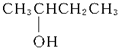 ��2-������
��2-������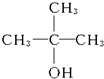 ��2-��-2-������
��2-��-2-������