��Ŀ����
10��ij���ʵķ���ʽΪC4H10O����1�����ܱ�������������̼ͬԭ������ȩ��2�֣����Ƕ�Ӧ�Ĵ��ֱ�Ϊ��д�ṹ��ʽ��CH3CH2CH2CH2OH��
 ��
����2�����ܱ�����������̼ͬԭ������ͪ��1�֣�д���ṹ��ʽ������
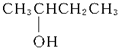 ��2-������
��2-��������3�����ܱ��������Ĵ��Ľṹ��ʽ������
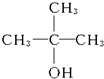 ��2-��-2-������
��2-��-2-��������4���ܷ�����ȥ��Ӧ���л�����ֻ��һ�����ʵ���3�֣����ֵ���1�֣�����Ľṹ��ʽΪCH2�TCHCH2CH3��CH3CH�TCHCH3��
���� ��1������ʽΪC4H10O���л���ܱ�������������̼ͬԭ������ȩ��������Ϊ��������д����-C4H9�칹�������칹��Ŀ���ڶ������칹����Ŀ��������������ȩ��˵�����ǻ�������̼����2����ԭ�ӣ�
��2������ʽΪC4H10O���л���ܱ�������������̼ͬԭ������ͪ��������Ϊ��������д����-C4H9�칹�������칹��Ŀ���ڶ������칹����Ŀ��������������ͪ��˵�����ǻ�������̼����1����ԭ�ӣ�
��3������ʽΪC4H10O���л�����ܱ��������Ĵ���������Ϊ��������д����-C4H9�칹�������칹��Ŀ���ڶ������칹����Ŀ�������ܱ�������˵�����ǻ�������̼��û����ԭ�ӣ�
��4������ʽΪC4H10O���л���ܷ�����ȥ��Ӧ��������Ϊ��������д����-C4H9�칹�������칹��Ŀ���ڶ������칹����Ŀ����������ȥ��Ӧ�Ľṹ�ص��ǣ�ֻ���ǻ�����̼������̼������ԭ�ӵIJ��ܷ�����ȥ��Ӧ���γɲ����ͼ���
��� �⣺��1������-C4H9���ܵĽṹ�У�-CH2CH2CH2CH3��-CH��CH3��CH2CH3��-CH2CH��CH3��2��-C��CH3��3���������ǻ�������̼����2����ԭ�ӵ��ǣ�-CH2CH2CH2CH3��-CH2CH��CH3��2�����Ը��л���Ŀ��ܽṹ��2�֣�CH3CH2CH2CH2OH�� ��
��
�ʴ�Ϊ��2��CH3CH2CH2CH2OH�� ��
��
��2������-C4H9���ܵĽṹ�У�-CH2CH2CH2CH3��-CH��CH3��CH2CH3��-CH2CH��CH3��2��-C��CH3��3���������ǻ�������̼����1����ԭ�ӵ��ǣ�-CH��CH3��CH2CH3�����Ը��л���Ŀ��ܽṹ��1�֣�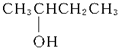 ��Ϊ2-������
��Ϊ2-������
�ʴ�Ϊ��1��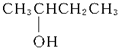 ��2-������
��2-������
��3������-C4H9���ܵĽṹ�У�-CH2CH2CH2CH3��-CH��CH3��CH2CH3��-CH2CH��CH3��2��-C��CH3��3���������ǻ�������̼��û����ԭ�ӵ��ǣ�-C��CH3��3�����Ը��л���Ŀ��ܽṹ��1�֣� ��Ϊ2-��-2-������
��Ϊ2-��-2-������
�ʴ�Ϊ�� ��2-��-2-������
��2-��-2-������
��4������-C4H9���ܵĽṹ�У�-CH2CH2CH2CH3��-CH��CH3��CH2CH3��-CH2CH��CH3��2��-C��CH3��3���������ǻ�����̼������̼������ԭ�ӵ��ǣ���-CH2CH2CH2CH3��-CH��CH3��CH2CH3��-CH2CH��CH3��2��-C��CH3��3�����Ը��л���Ŀ��ܽṹ��3�֣�HOCH2CH2CH2CH3��HOCH��CH3��CH2CH3��HOCH2CH��CH3��2��HOC��CH3��3����ȥ���������ֱ��У�1�֣�CH2�TCHCH2CH3����2�֣�CH2�TCHCH2CH3��CH3CH�TCHCH3����1�֣�CH2�TC��CH3��2����1�֣���CH3��2C�TCH2����
�ʴ�Ϊ��3�� 1��CH2�TCHCH2CH3��CH3CH�TCHCH3��
���� ���⿼���˴��Ĵ������Ĺ��ɣ���Ŀ�ѶȲ���ע�ⴼ��������ʵ�ʣ��ϼ���λ�ã�

| A�� | ���� ������ | B�� | ���� ������ | C�� | ���� ��ά�� | D�� | ���� �Ҵ� |
| A�� | H2C=CH-CH2-CH3 | B�� | 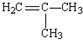 | C�� | H2C=CH2 | D�� | 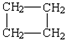 |
| A�� | ������ͬһԪ�� | B�� | һ����ͬһԪ�� | ||
| C�� | �˴�֮��һ����ͬλ�� | D�� | ���������һ����� |
 ����XԪ����Ԫ�����ڱ���λ�ڣ�������
����XԪ����Ԫ�����ڱ���λ�ڣ�������| A�� | ��������0�� | B�� | �������ڵڢ�A�� | C�� | �������ڵڢ�A�� | D�� | �������ڵڢ�A�� |
| A�� | Ԫ�����ڱ����߸����壬�˸����� | |
| B�� | IA���Ԫ��ȫ���ǽ���Ԫ�� | |
| C�� | Ԫ�����ڱ��ĵ����У��������ң�����Ԫ��������� | |
| D�� | Ԫ�����ڱ������������ڣ�����18��Ԫ�أ������������ڣ�����8��Ԫ�أ� |
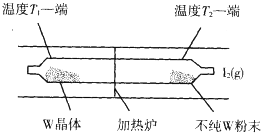 ���á���ѧ����ת�Ʒ����ᴿ�����ٵķ�Ӧԭ��Ϊ��W��s��+I2��g��?WI2��g����H��0
���á���ѧ����ת�Ʒ����ᴿ�����ٵķ�Ӧԭ��Ϊ��W��s��+I2��g��?WI2��g����H��0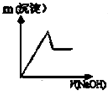

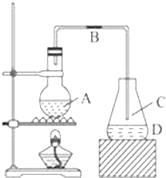 ij��ѧ����С������ͼװ����ȡ�屽���밴Ҫ���������С�⣺
ij��ѧ����С������ͼװ����ȡ�屽���밴Ҫ���������С�⣺ ��
��