��Ŀ����
��9�֣�������Ϊһ�������Դ�����������Ĵ������⣬ C60������������ϡ�
��1����֪���ʯ��C��C���ļ���Ϊ154.45 pm��C60��C��C���ļ���Ϊ145 pm��140 pm����ͬѧ�ݴ���ΪC60���۵���ڽ��ʯ������Ϊ�Ƿ���ȷ����������____________________________ ______��
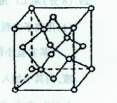
��2����ѧ�Ұ�C60��K������һ��������һ�ָ���ϩ������侧����ͼ��ʾ���������ڵ���ʱ��һ�ֳ����塣��������Kԭ�Ӻ�C60���ӵĸ�����Ϊ______________________��

��3����C60��ѧ���ֺϳ�Si60��N60��C��Si��Nԭ�ӵ縺���ɴ�С��˳����________ __��Si60������ÿ����ԭ��ֻ�����ڵ�3����ԭ���γɹ��ۼ�����ÿ����ԭ������㶼����8�����ȶ��ṹ����Si60�����Цм�����ĿΪ__________��
��1����֪���ʯ��C��C���ļ���Ϊ154.45 pm��C60��C��C���ļ���Ϊ145 pm��140 pm����ͬѧ�ݴ���ΪC60���۵���ڽ��ʯ������Ϊ�Ƿ���ȷ����������____________________________ ______��
��2����ѧ�Ұ�C60��K������һ��������һ�ָ���ϩ������侧����ͼ��ʾ���������ڵ���ʱ��һ�ֳ����塣��������Kԭ�Ӻ�C60���ӵĸ�����Ϊ______________________��

��3����C60��ѧ���ֺϳ�Si60��N60��C��Si��Nԭ�ӵ縺���ɴ�С��˳����________ __��Si60������ÿ����ԭ��ֻ�����ڵ�3����ԭ���γɹ��ۼ�����ÿ����ԭ������㶼����8�����ȶ��ṹ����Si60�����Цм�����ĿΪ__________��
��1������ȷ��C60�Ƿ��Ӿ��壬�ۻ�ʱ�����ƻ���ѧ�� ��3�֣�
��2��3��1�� ��2�֣� ��3��N��C��Si��30 ����2�֣�
��2��3��1�� ��2�֣� ��3��N��C��Si��30 ����2�֣�
��1�����ʯ��ԭ�Ӿ��壬��C60�Ƿ��Ӿ��壬�ۻ�ʱ�����ƻ���ѧ���������۵���ڽ��ʯ�ġ�
��2�����ݾ�����֪C60��8��λ�ڶ��㣬1�������ģ����Ը�����8��1/8��1��2����12����ԭ��ȫ��λ�����ϣ����Ը�����12��1/2��6����Kԭ�Ӻ�C60���ӵĸ�����Ϊ3��1��
��3���ǽ�����Խǿ���縺��Խ�����Ե縺���ɴ�С��˳����N��C��Si��Ҫ����ÿ����ԭ������㶼����8�����ȶ��ṹ�����ԭ���γɵ�3�����ۼ�����2���ǵ�����1��˫������ԭ�ӵļ۵�����4�������γɵ�˫������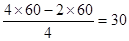 �����Ԧм�����Ŀ30��
�����Ԧм�����Ŀ30��
��2�����ݾ�����֪C60��8��λ�ڶ��㣬1�������ģ����Ը�����8��1/8��1��2����12����ԭ��ȫ��λ�����ϣ����Ը�����12��1/2��6����Kԭ�Ӻ�C60���ӵĸ�����Ϊ3��1��
��3���ǽ�����Խǿ���縺��Խ�����Ե縺���ɴ�С��˳����N��C��Si��Ҫ����ÿ����ԭ������㶼����8�����ȶ��ṹ�����ԭ���γɵ�3�����ۼ�����2���ǵ�����1��˫������ԭ�ӵļ۵�����4�������γɵ�˫������
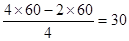 �����Ԧм�����Ŀ30��
�����Ԧм�����Ŀ30��
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ


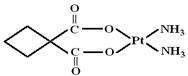
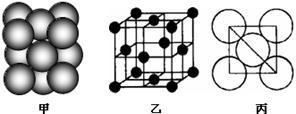
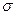 ����
����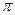 ������Ŀ֮��Ϊ
������Ŀ֮��Ϊ