��Ŀ����
20���ش��������⣮��ҵ��ˮ�г�����һ������Cr2O72-��CrO42-�����ǻ�����༰��̬ϵͳ�����ܴ���˺���������д��������õĴ��������л�ԭ���������÷��Ĺ�������Ϊ��

���еڢٲ�����ƽ�⣺2CrO42-����ɫ��+2H+�TCr2O72-����ɫ��+H2O
��1����ƽ����ϵ��pH=2������Һ�Գ�ɫ��
��2����˵���ڢٲ���Ӧ��ƽ��״̬����C��
A��Cr2O72-��CrO42-��Ũ����ͬ
B��2v ��CrO42-��=v ��Cr2O72-��
C����Һ����ɫ����
��3���ڢڲ��У���ԭ1mol Cr2O72-���ӣ���Ҫ6mol��FeSO4•7H2O��
���� ��1���������������ƽ���Ӱ����ȷ��ƽ���ƶ����Ӷ�ȷ������Ũ�ȴ�С����Һ��ɫ�仯��
��2�������ж�ƽ��״̬�ķ�����V��=V���������ֵ�Ũ�ȱ��ֲ�����˵���Ѵ�ƽ�⣻
��3������������ԭ��Ӧ�е�ʧ�����غ������㣮
��� �⣺��1��c��H+������ƽ��2CrO42-����ɫ��+2H+?Cr2O72-����ɫ��+H2O���ƣ���Һ�ʳ�ɫ���ʴ�Ϊ���ȣ�
��2�������ж�ƽ��״̬�ķ�����V��=V���������ֵ�Ũ�ȱ��ֲ�����˵���Ѵ�ƽ�⣬
A��Cr2O72-��CrO42-��Ũ����ͬȡ������ʼŨ�Ⱥ�ת���������ж�ƽ�⣬��A����
B��2v��Cr2O72-��=v��CrO42-���������ж����淴Ӧ���ʹ�ϵ�������ж�ƽ�⣬��B����
C����Һ����ɫ���䣬Ϊ�����������ж�ƽ�⣬��C��ȷ��
�ʴ�Ϊ��C��
��3�����ݵ��ӵ�ʧ�غ㣺n��Cr2O72-����6=n��FeSO4•7H2O����1��n��FeSO4•7H2O��=$\frac{1mol��6}{1}$=6mol���ʴ�Ϊ��6��
���� ������Ҫ�����˸����仯��������ʡ�������ԭ��Ӧ�������ܽ�ƽ��͵绯ѧ֪ʶ�����ݣ��Ѷ��еȣ�ץס��Ŀ��Ϣ�ǽ���Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
16�����з�Ӧ����������ԭ��Ӧ���������ȷ�Ӧ���ǣ�������
| A�� | ��Ƭ��ϡH2SO4��Ӧ | B�� | Ba��OH��2•8H20��NH4CI�ķ�Ӧ | ||
| C�� | ������02�е�ȼ�շ�Ӧ | D�� | ���ȵ�̿��C02��Ӧ |
8���������ʵ�ˮ��Һ�ܵ��磬�����ڷǵ���ʵ��ǣ�������
| A�� | FeCl3 | B�� | NH3 | C�� | NH4HCO3 | D�� | Cl2 |
15����nA���������ӵ�������nA������ֵ������˵����ȷ���ǣ�������
| A�� | ��״���£�22.4 L HCl����ˮ����Һ�к���nA��HCl���� | |
| B�� | 1L 0.1 mol•L-1��Na2SO4��Һ�к���0.2 nA��Na+ | |
| C�� | 1 mol ����������������������ΪnA | |
| D�� | ��0.1mol�Ȼ�������1Lˮ�У�������Һ����0.1nAFe3+ |
9�����ĺϳ�������Ҫ�Ļ�������֮һ��
��1����ҵ�Ͽ��ü�����ˮ��Ӧ�õ��ϳɰ��õ�H2�����Ȼ�ѧ��Ӧ����ʽΪCH4��g��+H2O��g��$\frac{\underline{\;����\;}}{\;}$CO��g��+3H2��g����H4����֪�йط�Ӧ�������仯��ͼ1��ʾ��
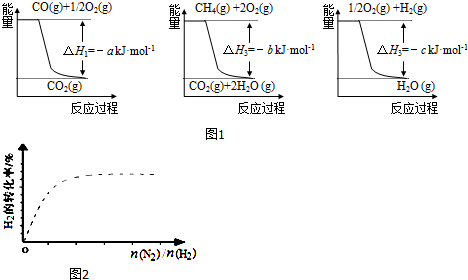
���H4=��a+3c-b��kJ•mol-1����a��b��c��ʾ��
��2����2����ѹ���ܱ������У�ͬ�¶��¡�ʹ����ͬ�����ֱ���з�Ӧ��3H2��g��+N2��g��$?_{����}^{���¸�ѹ}$2NH3��g��������ͬ��ʽͶ�뷴Ӧ����ֺ��£���Ӧ�ﵽƽ��ʱ�й�����Ϊ��
�ټ������ﵽƽ������Ҫ��ʱ��t=5min�����������������=������ͬ�����������ﵽƽ��ʱN2��Ũ��c=3mol•L-1��
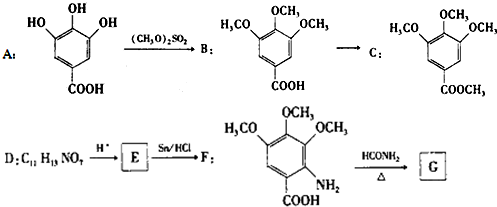
��ͼ2������Ϊ�÷�Ӧ��ʹ�ô��������£�������ʼN2��H2Ͷ�ϱȺ�H2ƽ��ת���ʵĹ�ϵͼ��������������ȫ��ͬʱ����ʵ������ʹ�ô��������H2ƽ��ת���ʵ�ʾ��ͼ��
��1����ҵ�Ͽ��ü�����ˮ��Ӧ�õ��ϳɰ��õ�H2�����Ȼ�ѧ��Ӧ����ʽΪCH4��g��+H2O��g��$\frac{\underline{\;����\;}}{\;}$CO��g��+3H2��g����H4����֪�йط�Ӧ�������仯��ͼ1��ʾ��

���H4=��a+3c-b��kJ•mol-1����a��b��c��ʾ��
��2����2����ѹ���ܱ������У�ͬ�¶��¡�ʹ����ͬ�����ֱ���з�Ӧ��3H2��g��+N2��g��$?_{����}^{���¸�ѹ}$2NH3��g��������ͬ��ʽͶ�뷴Ӧ����ֺ��£���Ӧ�ﵽƽ��ʱ�й�����Ϊ��
| �� �� | �� | �� |
| ��Ӧ��Ͷ���� | 3mol H2��2mol N2 | 6mol H2��4mol N2 |
| �ﵽƽ���ʱ�䣨min�� | t | 5 |
| ƽ��ʱN2��Ũ�ȣ�mol•L-1�� | 3 | c |
��ͼ2������Ϊ�÷�Ӧ��ʹ�ô��������£�������ʼN2��H2Ͷ�ϱȺ�H2ƽ��ת���ʵĹ�ϵͼ��������������ȫ��ͬʱ����ʵ������ʹ�ô��������H2ƽ��ת���ʵ�ʾ��ͼ��

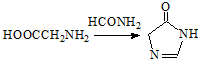
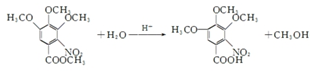 ��
��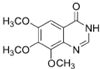 ��
��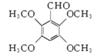 ��
��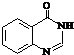 ���ϳɹ��������Լ���ѡ��
���ϳɹ��������Լ���ѡ�� ��
��