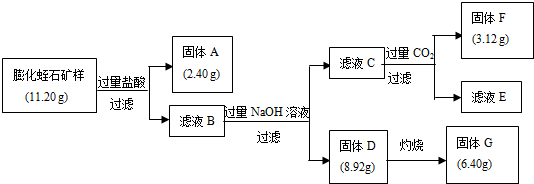
��Ŀ����
5��2009��9��27�գ��ȴ��籩�������ȡ��͡����ꡱ��ɷ��ɱ����ʡ�ݷ�����������ֺ�ɽ�廬�£�����500�����������ֹ�������ˮ������ɱ����Ϊ���ƴ��ģ��Ⱦ�Լ�����������Ч����֮һ��Ư���dz��õ�����������1���û�ѧ��Ӧ����ʽ��ʾ��ҵ����ȡƯ�۵�ԭ��2Ca��OH��2+2Cl2�TCaCl2+Ca��ClO��2+2H2O��
��2��Ư�۵ijɷ��ǣ��ѧʽ��CaCl2��Ca��ClO��2��
��3��Ư������ˮ���ܿ����е�CO2���ã�����Ư�ס�ɱ�����ã���ѧ��Ӧ����ʽΪCa��ClO��2+CO2+H2O=CaCO3��+2HClO��
���� ��1�������ڼ�����Һ����������������ԭ��Ӧ�������Ȼ���ʹ������Σ��Դ���д��ѧ����ʽ��
��2��Ư�۵ijɷ��Ǵ�����ơ��Ȼ��ƣ�
��3��Ư������ˮ���ܿ����е�CO2���ã����ɾ���Ư���Ե�HClO��
��� �⣺��1�������ڼ�����Һ����������������ԭ��Ӧ����ʯ���鷴Ӧ�����Ȼ��ơ�������ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪ2Ca��OH��2+2Cl2�TCaCl2+Ca��ClO��2+2H2O��
�ʴ�Ϊ��2Ca��OH��2+2Cl2�TCaCl2+Ca��ClO��2+2H2O��
��2����2Ca��OH��2+2Cl2�TCaCl2+Ca��ClO��2+2H2O��֪��Ư�۵ijɷ���CaCl2��Ca��ClO��2���ʴ�Ϊ��CaCl2��Ca��ClO��2��
��3���������������е�ˮ��CO2�������ɵĴ����ᣬ��Ӧ����ʽΪCa��ClO��2+CO2+H2O=CaCO3��+2HClO���ʴ�Ϊ��Ca��ClO��2+CO2+H2O=CaCO3��+2HClO��
���� ���⿼�����������ʼ�Ư�۵����ʣ�Ϊ��Ƶ���㣬�������ʵ����ʡ������ķ�Ӧ����ѧ������Ĺ�ϵΪ���Ĺؼ������ط�����Ӧ�������Ŀ��飬��Ŀ�ѶȲ���

��ϰ��ϵ�д�
 Сѧ��ʱ��ҵȫͨ����ϵ�д�
Сѧ��ʱ��ҵȫͨ����ϵ�д� �����ÿ�ʱѵ��ϵ�д�
�����ÿ�ʱѵ��ϵ�д�
�����Ŀ
20�������л����������ȷ���ǣ�������
| A�� | CH2��OH��CH2CH2��OH�� ������ | |
| B�� | ��CH3��3CCH2CH��C2H5��CH3 2��2��5-�������� | |
| C�� | 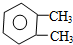 ���ױ� ���ױ� | |
| D�� | 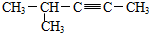 2-��-3-��Ȳ 2-��-3-��Ȳ |
10�������йػ�ѧ���������ǣ�������
| A�� | Nԭ�Ӽ۲�����ʾʽ��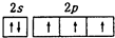 | |
| B�� | H2O����ʽΪ | |
| C�� | Ca2+���ӻ�̬�����Ų�ʽΪ1s22s22p63s23p6 | |
| D�� | �أ�K��ԭ�ӵ�ԭ�ӽṹʾ��ͼΪ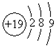 |
17�����й����л��������˵���У���ȷ���ǣ�������
| A�� | �л��ﶼ���ǵ���� | |
| B�� | ���е��л������ﶼ������ȼ�� | |
| C�� | �л�����һ������CԪ�أ�Ҳ�ɺ�H��N��S��Ԫ�� | |
| D�� | ���������͡��ƾ��������л��ܼ�������һ�����л������� |
14��ij��״�л�������к���m��-CH3��n��-CH2-��a��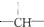 ������Ϊ-Cl����-Cl����Ŀ�����ǣ�������
������Ϊ-Cl����-Cl����Ŀ�����ǣ�������
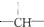 ������Ϊ-Cl����-Cl����Ŀ�����ǣ�������
������Ϊ-Cl����-Cl����Ŀ�����ǣ�������| A�� | 2n+3a-m | B�� | a+2-m | C�� | n+m+a | D�� | a+2n+2-m |
15�����������У�ǰ��һ�����ں��ߵģ�������
| A�� | 22.4LCO2��11.2LCO���������ķ����� | |
| B�� | ���ȷ�Ӧ�У���Ӧ������������������������ | |
| C�� | 0.2mol/LNaOH��0.1mol/LNa2CO3��Һ��Na+�����ʵ���Ũ�� | |
| D�� | 1molˮ������1mol笠������к��еĵ����� |