��Ŀ����
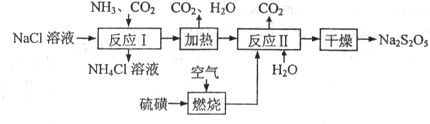
���������ƣ�Na2S2O5��������ʳƷƯ�������Ʊ������������£�

��֪���ٷ�Ӧ�����2NaHSO3?Na2S2O5+H2O�ȶಽ��Ӧ��
��Na2S2O5���ᷢ���ֽ⣺S2O52-+2H+=2SO2��+H2O
����Ҫ��ش��������⣺
��1��ʵ������ȡ�����Ļ�ѧ��Ӧ����ʽ��
��2�������£�0.1mol?L-1�Ȼ����ҺpH=5.1���������°�ˮ�ĵ��볣��Kb=10-x����x=
��3�������С����ȡ�ʱ������Ӧ�Ļ�ѧ����ʽΪ
��4��Ҫ�ӿ����������ȼ���ٶȣ����Բ�ȡ�Ĵ�ʩ��
��5����Ӧ������Ʋμӷ�Ӧ�Ĺ�������������ʵ���֮�Ƚӽ�
�����Ը�����أ� ��Ʒ����Һ�� �۳���ʯ��ˮ��
�ܱ���̼��������Һ����NaOH��Һ�� ��ϡ���ᣮ

��֪���ٷ�Ӧ�����2NaHSO3?Na2S2O5+H2O�ȶಽ��Ӧ��
��Na2S2O5���ᷢ���ֽ⣺S2O52-+2H+=2SO2��+H2O
����Ҫ��ش��������⣺
��1��ʵ������ȡ�����Ļ�ѧ��Ӧ����ʽ��
2NH4Cl+Ca��OH��2
CaCl2+2NH3��+2H2O
| ||
2NH4Cl+Ca��OH��2
CaCl2+2NH3��+2H2O
��
| ||
��2�������£�0.1mol?L-1�Ȼ����ҺpH=5.1���������°�ˮ�ĵ��볣��Kb=10-x����x=
4.8
4.8
�������֣�����1λС��������3�������С����ȡ�ʱ������Ӧ�Ļ�ѧ����ʽΪ
2NaHCO3
Na2CO3+CO2��+H2O
| ||
2NaHCO3
Na2CO3+CO2��+H2O
��
| ||
��4��Ҫ�ӿ����������ȼ���ٶȣ����Բ�ȡ�Ĵ�ʩ��
����Ƿ�����������ѹ���������������
����Ƿ�����������ѹ���������������
��дһ�֣�����5����Ӧ������Ʋμӷ�Ӧ�Ĺ�������������ʵ���֮�Ƚӽ�
1��2
1��2
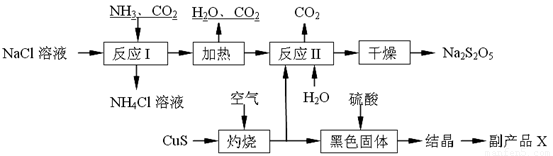
�������������㣬��ᵼ���������࣮�����Ʒ�к���̼�������������Լ����٢ڢۢ�٢ۢ�
�٢ڢۢ�٢ۢ�
������ţ��������Ը�����أ� ��Ʒ����Һ�� �۳���ʯ��ˮ��
�ܱ���̼��������Һ����NaOH��Һ�� ��ϡ���ᣮ
��������1��ʵ���������Ȼ�����ʯ�ҹ����Ʊ�������
��2��0.1mol?L-1�Ȼ����ҺpH=5.1��c��H+��=10-5.1��Kh=
=10-9.2��Kh��Kb=Kw��
��3�������ȡ�ʱ̼�����Ʒ�Ӧ����̼���ơ�ˮ�Ͷ�����̼��
��4���ӿ����������ȼ���ٶȣ�������Ӵ����������������Ũ�ȵȣ�
��5����Ӧ����Na2CO3+2SO2+H2O=2NaHSO3+CO2������������1��2��ȫ��Ӧ�����岻�����̼���ƣ����̼���Ƶ����ʷ�����
��2��0.1mol?L-1�Ȼ����ҺpH=5.1��c��H+��=10-5.1��Kh=
| 10-5.1��10-5.1 |
| 0.1 |
��3�������ȡ�ʱ̼�����Ʒ�Ӧ����̼���ơ�ˮ�Ͷ�����̼��
��4���ӿ����������ȼ���ٶȣ�������Ӵ����������������Ũ�ȵȣ�
��5����Ӧ����Na2CO3+2SO2+H2O=2NaHSO3+CO2������������1��2��ȫ��Ӧ�����岻�����̼���ƣ����̼���Ƶ����ʷ�����
����⣺��1��ʵ���������Ȼ�����ʯ�ҹ����Ʊ��������÷�ӦΪ2NH4Cl+Ca��OH��2
CaCl2+2NH3��+2H2O��
�ʴ�Ϊ��2NH4Cl+Ca��OH��2
CaCl2+2NH3��+2H2O��
��2��0.1mol?L-1�Ȼ����ҺpH=5.1��c��H+��=10-5.1��Kh=
=10-9.2��Kh��Kb=Kw��Kb=10-x������x=14-9.2=4.8���ʴ�Ϊ��4.8��
��3�������ȡ�ʱ̼�����Ʒ�Ӧ����̼���ơ�ˮ�Ͷ�����̼���÷�ӦΪ2NaHCO3
Na2CO3+CO2��+H2O���ʴ�Ϊ��2NaHCO3
Na2CO3+CO2��+H2O��
��4���ӿ����������ȼ���ٶȣ���ȡ�Ĵ�ʩΪ����Ƿ�����������ѹ��������������ȣ��ʴ�Ϊ������Ƿ�����������ѹ��������������ȣ�
��5����Ӧ����Na2CO3+2SO2+H2O=2NaHSO3+CO2������������1��2��ȫ��Ӧ�����岻�����̼���ƣ������Ʒ�к���̼�������ʼ��������������ø�����س��ӣ�������ʯ��ˮ����ǿɼ���̼���ƵĴ��ڣ������Լ��Ǣ٢ڢۢ�٢ۢޣ�
�ʴ�Ϊ��1��2���٢ڢۢ�٢ۢޣ�
| ||
�ʴ�Ϊ��2NH4Cl+Ca��OH��2
| ||
��2��0.1mol?L-1�Ȼ����ҺpH=5.1��c��H+��=10-5.1��Kh=
| 10-5.1��10-5.1 |
| 0.1 |
��3�������ȡ�ʱ̼�����Ʒ�Ӧ����̼���ơ�ˮ�Ͷ�����̼���÷�ӦΪ2NaHCO3
| ||
| ||
��4���ӿ����������ȼ���ٶȣ���ȡ�Ĵ�ʩΪ����Ƿ�����������ѹ��������������ȣ��ʴ�Ϊ������Ƿ�����������ѹ��������������ȣ�
��5����Ӧ����Na2CO3+2SO2+H2O=2NaHSO3+CO2������������1��2��ȫ��Ӧ�����岻�����̼���ƣ������Ʒ�к���̼�������ʼ��������������ø�����س��ӣ�������ʯ��ˮ����ǿɼ���̼���ƵĴ��ڣ������Լ��Ǣ٢ڢۢ�٢ۢޣ�
�ʴ�Ϊ��1��2���٢ڢۢ�٢ۢޣ�
���������⿼�����ʵ��Ʊ�ʵ�飬����ʵ���з����Ļ�ѧ��ӦΪ���Ĺؼ����漰����ˮ�⼰���㡢���ʵļ���ȣ��ۺ��Խ�ǿ����Ŀ�Ѷ��еȣ���5��Ϊ�����ѵ㣮

��ϰ��ϵ�д�
�����Ŀ
 Na2S2O5��H2O�ȶಽ��Ӧ��
Na2S2O5��H2O�ȶಽ��Ӧ��
 Na2S2O5��H2O�ȶಽ��Ӧ��
Na2S2O5��H2O�ȶಽ��Ӧ��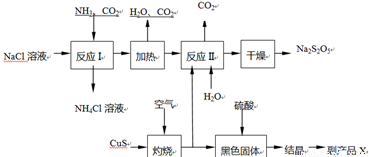
 Na2S2O5��H2O�ȶಽ��Ӧ��
Na2S2O5��H2O�ȶಽ��Ӧ��