��Ŀ����
16���Ϻ�ijС���ϣ������õĵ�ˮ��Դ�൱�ѷ�����ž�սʿΪ��Ѱ�Һ��ʵ�����ˮԴ���Ե���ɽȪˮ���з������飬�����ʾɽȪˮ����Ӳˮ����1��Ӳˮ��ָ���н϶�Ca2+��Mg2+��ˮ��Ӳˮ���Ⱥ�������������ӷ���ʽΪ��Ca2++2HCO3-$\frac{\underline{\;\;��\;\;}}{\;}$CaCO3��+CO2��+H2O��д������һ�ֳ�����ļ��ɣ���
��2��Ӳ��Ϊ1���ˮ��ָÿ��ˮ��10mg CaO����֮�൱�����ʣ���7.1mg MgO������֪ˮ��Ӳ����8�����µ���ˮ����8�����ϵij�ΪӲˮ����֪����ɽȪˮ��c��Ca2+��=1.2��10-3mol/L��c��Mg2+��=6��l0-4 mol/L����ô��ˮ�ǣ���ǡ�������Ӳˮ��
��3�����ӽ�����������ˮ�ij��÷������۱�ϩ������һ�����ӽ�����֬��д���۱�ϩ���Ƶ���Ľṹ��ʽCH2=CHCOONa��
��4����ž�սʿͨ����ˮ�м���������ˮ���������ӷ���ʽ�����侻ˮԭ����Al3++3H2O?Al��OH��3�����壩+3H+��
��5�����ϻ������ú�ˮ��������õ�ˮ����ͼ�Ǻ�ˮ���õ���������õ�ˮ��ԭ��ͼ����֪��ˮ�к�Na+��Cl-��Ca2+��Mg2+��SO42-�����ӣ��缫Ϊ���Ե缫��������������⣺
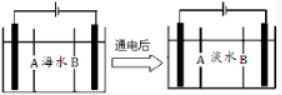
�������ӽ���Ĥ��ָB����A��B����
��д��ͨ����������ĵ缫��Ӧʽ2Cl--2e-�TCl2�����������������ǣ��缫�ϲ������ݣ���Һ�г���������ɫ������
���� ��1��Ӳˮ�Ǻ��н϶�����Ը��Ρ�þ�ε�ˮ��Ӳˮ�е�̼��������ȷֽ�����̼��Ƴ�����
��2������Ӳ��Ϊ1���ˮ��ָÿ��ˮ��10mgCaO����֮�൱�����ʣ���7.1mgMgO������ˮ�е�Ca2+��Mg2+���������CaO����������õ���
��3���۱�ϩ���Ƶĵ���Ϊ��ϩ�ƣ�
��4��������ˮ���������������������ӣ��������������������ܹ��������ʿ���������������ˮ��
��5���������ӽ���Ĥֻ��������������ͨ���������ӽ���Ĥֻ��������������ͨ����
�ڸ��������������ӷŵ磬�������������ӵõ�������������������������Ũ������
��� �⣺��1��Ӳˮ�Ǻ��н϶������Ca2+��Mg2+��ˮ��Ӳˮ�е�̼��������ȷֽ�����̼��Ƴ�������Ӧ�����ӷ���ʽΪ��Ca2++2HCO3-$\frac{\underline{\;\;��\;\;}}{\;}$CaCO3��+CO2��+H2O��
�ʴ�Ϊ��Ca2+��Mg2+��Ca2++2HCO3-$\frac{\underline{\;\;��\;\;}}{\;}$CaCO3��+CO2��+H2O��
��2��ij��Ȼˮ��c��Ca2+��=1.2��10-3mol•L-1��c��Mg2+��=6��10-4mol•L-1��Ӳ��Ϊ1���ˮ��ָÿ��ˮ��10mgCaO����֮�൱�����ʣ���7.1mgMgO����1Lˮ�и��������ʵ���=1.2��10-3mol���൱��CaO������1.2��10-3mol��56g/mol=67.2mg��1Lˮ��þ�������ʵ���=6��10-4mol���൱������þ������6��10-4mol��40g/mol=24mg��ˮ��Ӳ��=$\frac{67.2mg}{10mg}$+$\frac{24mg}{7.1mg}$=10�㣬����Ӳˮ��
�ʴ�Ϊ���ǣ�
��3���۱�ϩ���Ƶĵ���Ϊ��ϩ�ƣ��ṹ��ʽ��CH2=CHCOONa���ʴ�Ϊ��CH2=CHCOONa��
��4��������ˮ���������������������ӣ����ӷ���ʽ��Al3++3H2O?Al��OH��3�����壩+3H+���ʴ�Ϊ��Al3++3H2O?Al��OH��3�����壩+3H+��
��5���������ӽ���Ĥֻ��������������ͨ���������ӽ���Ĥֻ��������������ͨ������ĤB�����������������������ӷŵ磬���Ը�ĤB�������ӽ���Ĥ��
�ʴ�Ϊ��B��
�ڸ��������������ӷŵ磺2Cl--2e-�TCl2�����������������ӵõ�������������������������Ũ���������ӣ�þ�����γɳ�����
�ʴ�Ϊ��2Cl--2e-�TCl2�����缫�ϲ������ݣ���Һ�г���������ɫ������
���� ���⿼����ԭ��ؼ��乤��ԭ����Ӧ�ã���Ŀ�Ѷ��еȣ��漰Ӳˮ����������������ˮ��ԭ��ع���ԭ����֪ʶ����ȷ��ظ�����ԭ���ǽ���ؼ�������������ѧ�������Ӧ��������

| ��ѧʽ | CH3COOH | H2CO3 | HClO | |
| ����ƽ�ⳣ�� | 1.8��10-5 | K1��4.3��10-7 K2��5.6��10-11 | 3.0��10-8 | |
��1�������������ӽ�����ӵ������ɴ�С��˳����a��b��d��c�������ţ�
a��CO32- b��ClO- c��CH3COO- d��HCO3-��
��2�����з�Ӧ���ܷ������ǣ�cd
a��CO32-+CH3COOH�TCH3COO-+CO2��+H2O
b��ClO-+CH3COOH�TCH3COO-+HClO
c��CO32-+HClO�TCO2��+H2O+ClO-
d.2ClO-+CO2+H2O�TCO32-+2HClO
��3��������ˮϡ��0.10mol•L-1�Ĵ��ᣬ�����и�ʽ��ʾ����ֵ��ˮ�������Ӷ��������B
A��$\frac{c��C{H}_{3}COOH��}{c��{H}^{+}��}$ B��$\frac{c��C{H}_{3}CO{O}^{-}��}{c��C{H}_{3}COOH��}$
C��$\frac{c��{H}^{+}��}{{K}_{W}}$ D��$\frac{c��{H}^{+}��}{c��O{H}^{-}��}$
��4�����Ϊ10mL pH=2�Ĵ�����Һ��һԪ��HX�ֱ��ˮϡ����1 000mL��ϡ����pH�仯 ��ͼ�ף���HX�ĵ���ƽ�ⳣ�����ڣ�����ڡ��������ڡ���С�ڡ��������ƽ�ⳣ����ϡ�ͺ�HX��Һ��ˮ���������c��H+�����ڴ�����Һ��ˮ���������c��H+��������ڡ��������ڡ���С�ڡ���

��5����20mL���������Ļ����Һ�У���μ���0.05mol•L-1Ba��OH��2��Һʱ�����ɳ����������仯���ɴ˶��������Һ��pH�ı仯��ͼ����ʾ�����㣺
��ԭ�����Һ��c��H+��=0.3mol/L��c��Cl-��=0.2mol/L��
��A���pH=1��
�۽�0.15mol•L-1ϡ����V1mL��0.1mol•L-1NaOH��ҺV2mL��ϣ�������Һ��pHΪ1����V1��V2=1��1����Һ����仯���Բ��ƣ���
| A�� | m+n��p | B�� | C����������½� | ||
| C�� | ƽ�����淴Ӧ�����ƶ� | D�� | A��ת���ʱ�� |
| A�� | ��Һ�dzʵ����Եģ��������ǿ��Դ���� | |
| B�� | ���ˮ�м���FeCl3������Һ�������������Һ�ʺ��ɫʱ���õ�Fe��OH��3���� | |
| C�� | �峿����������Ҷ��ķ�϶���Բ��������ЧӦ��˵��������һ�ֽ��� | |
| D�� | ������������Һ�ͽ���ķ��������ö����ЧӦ�����ڻ�ѧ���� |
���������� �����ᱵ ��ͭ ������ �ݶ�������
| A�� | �٢� | B�� | �٢ڢ� | C�� | �ۢ� | D�� | �٢� |
| A�� | ��ҵ����ˮ����NO2�������3NO2+H2O�T2HNO3+NO | |
| B�� | �ð�ˮ��ȥ��ҵԭ���Ȼ���е��Ȼ������ʣ�Fe3++3OH-�TFe��OH��3�� | |
| C�� | ����ʯ�Ҵ���й©��Һ�ȣ�2Ca��OH��2+2Cl2�TCaCl2+Ca��ClO��2+2H2O | |
| D�� | ��������ȥˮ�е����������Al3++3H2O?Al��OH��3 �����壩+3H+ |
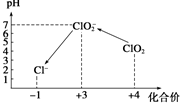 ClO2������һ����ˮ�����ȷ����й㷺Ӧ�õĸ�Ч��ȫ���������ҹ���2 000��������ClO2��������������ˮ����������
ClO2������һ����ˮ�����ȷ����й㷺Ӧ�õĸ�Ч��ȫ���������ҹ���2 000��������ClO2��������������ˮ����������