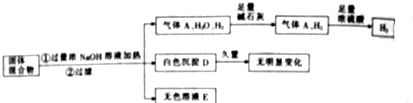
��Ŀ����
14��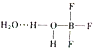 ��Ԫ���ڻ�ѧ�к���Ҫ�ĵ�λ�����仯����㷺Ӧ����ˮ�Ų��ϡ��������ϡ���ȼ�ϲ��ϡ����ϲ��ϵȸ��²�������Ӧ�ã�
��Ԫ���ڻ�ѧ�к���Ҫ�ĵ�λ�����仯����㷺Ӧ����ˮ�Ų��ϡ��������ϡ���ȼ�ϲ��ϡ����ϲ��ϵȸ��²�������Ӧ�ã���1�����������ڳ��³�ѹ��Ϊ���д̱Ƕ����ǿ�̼��Ե���ɫ�ж���ʴ�����壬����ӵ����幹��Ϊƽ�������Σ�Bԭ���ӻ�����Ϊsp2��
��2��BF3��ˮһ�����γɾ��壨H2O��2•BF3������ṹ��ͼ�������и������������������漰��
����λ�� ����� �۷��»��� �ܼ��Թ��ۼ� �ݷǼ��Թ��ۼ���
������ȷ���Ǣ٢ڢۢܣ�
��3��NH4BF4��������泥��Ǻϳ������ܵ�ԭ��֮һ��1mol NH4BF4����2mol��λ����
���� ��1�������ж�����ԭ���γɵĦļ���Ŀ��Ȼ���жϹ¶Ե�����Ŀ���Դ��ж��ӻ����ͣ���ϼ۲���ӶԻ���ģ�Ϳ��жϷ��ӵĿռ乹�ͣ�
��2�����пչ����ԭ�Ӻͺ��йµ��ӶԵ�ԭ��֮�����γ���λ��������֮����ڷ��»�����ˮ�����е�Oԭ�Ӻ����������е�Hԭ�����γ��������ͬ�ǽ���ԭ��֮���γɵĹ��ۼ�Ϊ�Ǽ��Թ��ۼ�����ͬԭ��֮���γɵ�Ϊ���Թ��ۼ���
��3��һ��NH4BF4��Nԭ�Ӻ�����һ��Hԭ��֮�������λ����Bԭ�Ӻ�����һ��Fԭ��֮�����һ����λ�������Ժ���2����λ�����ݴ˼��㣮
��� �⣺��1��BF3��Bԭ���γ�3���ļ����¶Ե�����Ϊ$\frac{3-3��1}{2}$=0��BF3�м۲���ӶԸ���=3+$\frac{1}{2}$����3-3��1��=3���Ҳ����µ��Ӷԣ����Կռ乹����ƽ�������Σ�����ԭ������sp2�ӻ���
�ʴ�Ϊ��ƽ�������Σ�sp2��
��2�������ԭ��֮�����γɼ��Թ��ۼ���Bԭ�Ӻ��пչ����Oԭ�Ӻ��йµ��Ӷԣ�����Bԭ�Ӻ�Oԭ��֮�������λ��������֮����ڷ��»�����ˮ�����е�Oԭ�Ӻ����������е�Hԭ�����γ���������Բ��漰���ǷǼ��Թ��ۼ���
�ʴ�Ϊ���٢ڢۢܣ�
��3��һ��NH4BF4��Nԭ�Ӻ�����һ��Hԭ��֮�������λ����Bԭ�Ӻ�����һ��Fԭ��֮�����һ����λ�������Ժ���2����λ������1mol NH4BF4����2mol��λ����
�ʴ�Ϊ��2��
���� ���⿼�������ʽṹ�����ʣ��漰���ӵ����幹�͡��ӻ�����λ���Ŀ��飬������λ���ĸ���۲���ӶԻ������۵�֪ʶ�������������Ŀ�Ѷ��еȣ�

| A�� | Cl2��Fe3+��I2��S | B�� | S��I2��Fe3+��Cl2 | C�� | Fe3+��Cl2��H2S��I2 | D�� | Cl2��I2��Fe3+��H2S |
| A�� | 1mol Fe����1molˮ������ַ�Ӧת�Ƶĵ�����Ϊ3NA | |
| B�� | ��⾫��ͭʱ����ת����NA�����ӣ�����������32 gͭ | |
| C�� | 6.8�����ڵ�KHSO4���к���0.1NA�������� | |
| D�� | ��״���£�11.2L���Ȼ�̼����������Ϊ0.5NA |
| A�� | ��X��Y��ȫȼ�պ�����CO2��H2O�����ʵ���֮�Ⱦ�Ϊ1��1 | |
| B�� | ����������X��Y��ȫȼ��ʱ����O2����һ����� | |
| C�� | �������ʵ�����X��Y��ȫȼ��ʱ����O2����һ����� | |
| D�� | ����X��Y�Ժ��ֱ�����ϣ�ֻҪ������һ������ȫȼ��ʱ���ĵ�O2����һ����� |
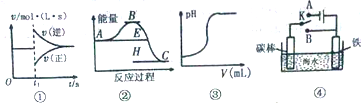
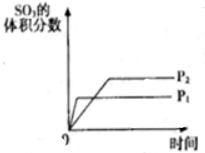
| A�� | ͼ�ٿ��Ա�ʾ�����淴Ӧ2SO2��g��+O2��g��?2SO3��g����H��0ά���¶ȡ�ѹǿ���䡢t1ʱ����SO3��g�� | |
| B�� | ����ͼ���жϣ�����A��Ӧ������C�ġ�H��0 | |
| C�� | ͼ�۱�ʾ��һ����������������Һ�еμ�һ��Ũ��������Һʱ��pH�仯 | |
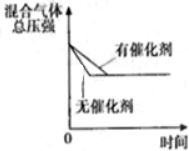
| D�� | ͼ���п���K����A��B�����ɼ������ĸ�ʴ |
| A�� | �������᳧β���е�SO2��SO2+2NH3+H2O�T��NH4��2SO3 | |
| B�� | ���������Ṥҵβ���е��������NO2+NO+2NaOH�T2NaNO2+H2O | |
| C�� | ��CuSO4��Cu+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CuSO4+SO2��+2H2O | |
| D�� | ��CuSO4��2Cu+O2$\frac{\underline{\;\;��\;\;}}{\;}$2CuO��CuO+H2SO4��ϡ���TCuSO4+H2O |
| A�� | �ü���ʼ���ijKI��Һ���Ƿ����е��� | |
| B�� | ���ȿɳ�ȥNaHCO3�����е�Na2CO3���� | |
| C�� | ���ˮ�м��뱽��ƾ������ɴӵ�ˮ����ȡ�� | |
| D�� | 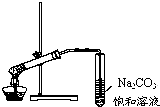 ����ͼ��ʾ��װ����ȡ�������������� |