��Ŀ����
����Ŀ�������������������ʻ�����ijѧϰС��ͨ��ʵ��̽��һ�ֵ����ʵ�Ԫ����ɡ�
I.ȷ���õ������е�ijЩ���Ԫ��
(1)Ϊȷ���õ������к���Ԫ�أ�Ҫ����Ʒ���л���ת������Ρ���֤��笠����ڵ����ӷ���ʽ��__��
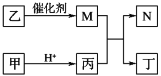
(2)Ϊȷ���õ������к�̼���⡢������Ԫ�أ�������ͼװ�ý���̽����ͨ������ʹ��Ʒ��װ��A�г��ȼ�գ���ʹ��������λ���ͨ������װ�á�

��װ��B�е��Լ���________��
��װ��D��������_______��
�۵�װ��B��C��E��F���γ�����������_____��Ʒ����Һ��ɫ��_____�����ְ�ɫ���ǣ���֤��ȼ�ղ����к���H2O��SO2��CO2�����ۣ��õ������к�̼���⡢����Ԫ�ء�
II.Ϊ�ⶨ�õ���������Ԫ�ص�����������С��ȡ��������Ʒ���ȼ�գ�����������ˮ���ն���������ȡ����Һ���Ե���Ϊָʾ�����������������Һ�ζ������ĵ⣬��֪��2![]() ��I2===
��I2===![]() ��2I-��
��2I-��
(3)д�������������ˮ��Ӧ�Ļ�ѧ����ʽ��_______��
(4)�ﵽ�ζ��յ�ı�־Ϊ________��
(5)ȡ��������Ʒm g���вⶨ������c1 mol��L-1�ĵ�ˮV1 mL�������գ��ζ������ĵ���ʱ����c2 mol��L-1�����������ҺV2 mL���õ������е���Ԫ�ص���������Ϊ______��
(6)��ȼ��ʱ����������������Һ�У����ܻᵼ�¸õ������е���Ԫ�ص����������ⶨֵ______(����ƫ������ƫС��������Ӱ����)��
���𰸡�![]() +OH-
+OH- ![]() NH3��+H2O ��ˮ����ͭ ��ȥSO2 ��ɫ��ĩ����ɫ Ʒ����Һ����ɫ I2��SO2��2H2O =H2SO4��2HI ���������һ��Na2S2O3��Һ����Һ����ɫ��ȥ���Ұ�����ڲ��ָ�ԭɫ
NH3��+H2O ��ˮ����ͭ ��ȥSO2 ��ɫ��ĩ����ɫ Ʒ����Һ����ɫ I2��SO2��2H2O =H2SO4��2HI ���������һ��Na2S2O3��Һ����Һ����ɫ��ȥ���Ұ�����ڲ��ָ�ԭɫ ![]() ƫС
ƫС
��������
(1)֤����δ��ڣ�һ��ͨ�������ת��ΪNH3������֤��ʵ���������������������Һ�����ȣ�������ʹʪ��ļtɫʯ����ֽ����ɫ�����壬���ӷ���ʽΪ![]() +OH-
+OH- ![]() NH3��+H2O���ʱ����Ϊ��
NH3��+H2O���ʱ����Ϊ��![]() +OH-
+OH- ![]() NH3��+H2O��
NH3��+H2O��
(2)��װ��B�������Ǽ��鵰���ʵ�ȼ�ղ����к���H2O��װ��Bʢ�ŵ��Լ�Ϊ��ˮ����ͭ���ʴ�Ϊ����ˮ����ͭ��
��װ��C�������Ǽ���SO2��װ��D�������dz�ȥSO2�������CO2�ļ���������ţ�װ��E�������Ǽ���SO2�Ƿ������װ��F�������Ǽ���CO2���ʱ����Ϊ����ȥSO2��
��֤��ȼ�ղ����к���H2O������Ϊװ��B�а�ɫ��ĩ��Ϊ��ɫ��֤������SO2������Ϊװ��C��Ʒ����Һ��ɫ��֤������CO2��������װ��E��Ʒ�첻��ɫ��װ��F�г��ְ�ɫ���ǣ��ʱ����Ϊ����ɫ��ĩ����ɫ��Ʒ����Һ����ɫ��
(3)SO2��I2������Ӧ��SO2������ΪH2SO4��I2����ԭΪI-����ѧ����ʽΪ��I2��SO2��2H2O =H2SO4��2HI���ʴ�Ϊ��I2��SO2��2H2O =H2SO4��2HI��
(4)���������ʵ����ɫ����ζ��յ�ʱ��I2ת��ΪI-��������Ϊ�������һ��Na2S2O3��Һ����Һ����ɫ��ȥ���Ұ�����ڲ��ָ�ԭɫ���ʱ����Ϊ�����������һ��Na2S2O3��Һ����Һ����ɫ��ȥ���Ұ�����ڲ��ָ�ԭɫ��
(5)����I2��SO2��2H2O =H2SO4��2HI��2![]() ��I2===
��I2===![]() ��2I-����n(I2)=n(SO2)+
��2I-����n(I2)=n(SO2)+![]() n(Na2S2O3)����n(SO2)= n(I2)-
n(Na2S2O3)����n(SO2)= n(I2)-![]() n(Na2S2O3)=c1V1��10-3mol-0.5c2V2��10-3mol����õ������е���Ԫ�ص���������Ϊ
n(Na2S2O3)=c1V1��10-3mol-0.5c2V2��10-3mol����õ������е���Ԫ�ص���������Ϊ![]() =
=![]() ���ʱ����Ϊ��
���ʱ����Ϊ��![]() ��
��
(6)��ȼ��ʱ����������������Һ�У��ᷢ����Ӧ��2SO2+O2+2H2O=2H2SO4��������Ԫ�ص����������ⶨֵƫС���ʴ�Ϊ��ƫС��

����Ŀ���й��Ŵ����������̺��˷ḻ�Ļ�ѧ֪ʶ����ؼ��ز������顣���ж�����������ؼ��ص�ԭ�����Ͳ���ȷ����
ѡ�� | ��ؼ��� | ���� |
A. | �������ӡ��������ࣨCuSO4��Ϳ��������ɫ��ͭ���������ڲ���Ҳ���� | ���������û�ͭ���ʵķ�Ӧ |
B. | �����ϴ�������ࡷ�����𣨹Ŵ�������������ָͭ�������ᣬ��������ա��� | �Ͻ��Ӳ�ȴ���������Ĵ� ������Ӳ�� |
C. | �������ӡ�������ɰ��HgS����֮��ˮ���������ֻ��ɵ�ɰ���� | �����˿��淴Ӧ��HgS |
D. | �����ײ�ͬ������������[2PbCO3��Pb(OH)2]Ͷ���У�ɫ����ΪǦ��Pb������ | �˴����������к���̿���� ���۷����ֽⷴӦ���ɵ�Ǧ�������ﻹԭΪ����Ǧ�� |
A.A B.BC.CD.D
����Ŀ���������γɵĻ���������࣬�ճ�������Ӧ�ù㷺�������������(Na2S2O3)����Ϊ����ҵ�Ķ�Ӱ������Ӧ�Ļ�ѧ����ʽ���£�AgBr��2Na2S2O3===Na3[Ag(S2O3)2]��NaBr���ش��������⣺
(1)��̬S�ļ۵����Ų�ͼΪ____________��
(2)���й������ʽṹ�����ʵ�˵������ȷ����________��
A������ԭ�ӽṹģ���ܹ��ɹ��ؽ�����ԭ�ӹ���
B��Br��S��O����Ԫ�صĵ縺��˳��Ϊ O��Br��S
C��Na�ĵ�һ������С�� Mg������ڶ�������ȴԶ���� Mg
D��ˮ���Ӽ�����������H2O���۷е㼰�ȶ��Ծ�����H2S
(3)����VSEPR�����Ʋ�![]() �Ŀռ乹��Ϊ______������ԭ��S���ӻ���ʽΪ________��[Ag(S2O3)2]3-�д��ڵĻ�ѧ����________(����ĸ)��
�Ŀռ乹��Ϊ______������ԭ��S���ӻ���ʽΪ________��[Ag(S2O3)2]3-�д��ڵĻ�ѧ����________(����ĸ)��
A�����Ӽ� B�����Լ� C���Ǽ��Լ� D�������� E����λ��
(4)��һ��������(E1)��ָԪ�صĻ�̬��̬ԭ�ӵõ�һ�������γ���̬��һ������ʱ���ų�������(��λΪkJ��mol-1)����������Խ��Ԫ��ԭ��Խ�õ��ӡ���֪�������ڲ���Ԫ�ص�һ�����������±���
Ԫ�� | Al | Si | P | S | Cl |
E1/(kJ��mol-1) | 42.5 | 134 | 72.0 | 200 | 349 |
����Ԫ�ص�E1������ҳ��������ƣ��Է���PԪ�س����쳣��ԭ��___________��
(5)ij�����������������ᄃ����ͼ��ʾ������A��B��ɡ����������Ļ�ѧʽΪ_____����֪�þ���ľ�������Ϊa nm�������ӵ�������ֵΪNA�����ܶ���Ϊ______g��cm-3(�ú�a��NA �Ĵ���ʽ��ʾ)��