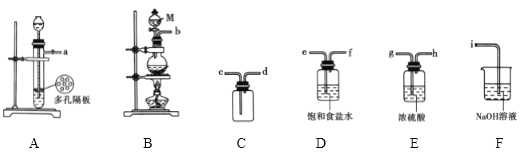
��Ŀ����
����Ŀ��1,2������������Ʊ�ԭ���ǣ�CH3CH2OH![]() CH2=CH2��+H2O CH2=CH2+Br2��BrCH2��CH2Br��ij����С������ͼ��ʾ��װ���Ʊ�1,2���������顣
CH2=CH2��+H2O CH2=CH2+Br2��BrCH2��CH2Br��ij����С������ͼ��ʾ��װ���Ʊ�1,2���������顣

�ش��������⣺
(1)װ��B��������_______��
(2)������ƿ�ڼ���һ�������Ҵ���Ũ������Һ��������ɰ��������ɰĿ����_______������E��������_______��
(3)װ��C�ڷ�������Ҫ��Ӧ�����ӷ���ʽΪ_______��
(4)����������ƿǰ���Ƚ�C��D���Ӵ��Ͽ����ٽ�������ƿ��ʯ�����ϼ��ȣ����¶�����Լ120��ʱ������C��D����Ѹ�ٽ�A�ڷ�Ӧ�¶�������160��180�棬�ӵ�Һ©���������μ��Ҵ���Ũ������Һ��������ϩ������ȵ�ͨ��װ��3.20mLҺ��(��Һ��=3g/cm3)��3mLˮ��D���Թܣ�ֱ����Ӧ������
�ٽ�C��D���Ӵ��Ͽ���ԭ����_______
���жϷ�Ӧ������������_______
(5)����Ʒ�����Һ©�����ֱ���ˮ������������Һ��ˮϴ�ӣ���Ʒ����ˮ�Ȼ��Ƹ�����˺������ռ�129��133����֣��õ�7.896g1,2���������顣1,2����������IJ���Ϊ_______��
(6)���в����У����ᵼ�²�����ʽ��͵���(����ȷ�𰸵ı��)_______��
a.��ϩͨ����ˮʱ����̫�� b��װ��E�е�NaOH��Һ��ˮ���森
c.ȥ��װ��D�ձ��е�ˮ d.ʵ��ʱû��Cװ��
e��D�е��Թ��ﲻ��ˮ
���𰸡���ȫƿ������ ������ ��ƿ SO2+2OH��=SO32��+H2O �����������ˮ�����������������Ļӷ� D���Թܵ���ˮ��ɫ(���Թ����Һ���Ϊ��ɫ) 70% b
��������
ʵ�����Ʊ�1��2-�������飺������ƿA�з�����Ӧ���Ҵ���Ũ����������·�����������ˮ��ȡ��ϩ���Ҵ���������ȥ��Ӧ����Ӧ����ʽΪ��CH3CH2OH![]() CH2=CH2��+H2O�����D�е����ܷ��������¹ʣ�A�в�������ϩ����ᵼ��װ��B��ѹǿ��������Һ�������������װ��B�г������ܿ��ж�װ���Ƿ������װ��B�����ã�Ũ���������ˮ�ԡ���ˮ�Ժ�ǿ�����ԣ��������Ҵ����������ɵ���������Ϊ������������̼��װ��C�з�����������Һ��������ӦSO2+2NaOH=Na2SO3+H2O��CO2+2NaOH�TNa2CO3+H2O����ȥ�������壬��ϩ���в����ͼ�C=C˫��������±�ص��ʷ����ӳɷ�Ӧ��D����ϩ����ӳ�����1��2-�������飬��ӦΪ��CH2=CH2+Br2��BrCH2��CH2Br���Ƶ�1��2-�������顣
CH2=CH2��+H2O�����D�е����ܷ��������¹ʣ�A�в�������ϩ����ᵼ��װ��B��ѹǿ��������Һ�������������װ��B�г������ܿ��ж�װ���Ƿ������װ��B�����ã�Ũ���������ˮ�ԡ���ˮ�Ժ�ǿ�����ԣ��������Ҵ����������ɵ���������Ϊ������������̼��װ��C�з�����������Һ��������ӦSO2+2NaOH=Na2SO3+H2O��CO2+2NaOH�TNa2CO3+H2O����ȥ�������壬��ϩ���в����ͼ�C=C˫��������±�ص��ʷ����ӳɷ�Ӧ��D����ϩ����ӳ�����1��2-�������飬��ӦΪ��CH2=CH2+Br2��BrCH2��CH2Br���Ƶ�1��2-�������顣
(1)һ�����巢��������װ��B�ڵ�Һ����뵽���������ڣ���ȫƿ�����ã�
��ˣ�������ȷ���ǣ���ȫƿ��������
(2)��Һ����ȣ��ײ���������������������ƿ�ڼ���һ�������Ҵ���Ũ������Һ��������ɰ��������ɰĿ���Ƿ����У�����E����������ƿ��
��ˣ�������ȷ���ǣ������У���ƿ��
(3)�Ҵ���Ũ���ᷢ����Ӧ���Ʊ�������ϩ�����к��������������������˻������ͨ������������Һ�������˶��������ᴿ����ϩ����������������������Һ��Ӧ�����������ƺ�ˮ�����ӷ���ʽΪSO2+2OH��=SO32��+H2O ��
��ˣ�������ȷ���ǣ�SO2+2OH��=SO32��+H2O��
(4) �ټ���������ƿǰ���Ƚ�C��D���Ӵ��Ͽ�������Ͳ��ܽ��뵽װ�����װ���ڣ������������Ļӷ���
��ˣ�������ȷ���ǣ������������ˮ�����������������Ļӷ���
����ϩ���巢���ӳɷ�Ӧ������ɫ�����������D���Թܵ���ˮ��ɫ�÷�Ӧ������
��ˣ�������ȷ���ǣ�D���Թܵ���ˮ��ɫ��
(5)������ʵ���Ϊ![]() =0.06mol�����ݷ�Ӧ��CH2=CH2+Br2��Br-CH2-CH2-Br��֪������1��2-�����������Ϊ0.06mol������Ϊ0.06mol��188g/mol=11.28g��
=0.06mol�����ݷ�Ӧ��CH2=CH2+Br2��Br-CH2-CH2-Br��֪������1��2-�����������Ϊ0.06mol������Ϊ0.06mol��188g/mol=11.28g��
��1,2-��������IJ���Ϊ![]() ��100%=70%��
��100%=70%��
��ˣ�������ȷ���ǣ�70%��
(6) a.ͨ���ٶȹ��죬��Ӧ����֣������²��ʽ��ͣ�a�������⣻
b.װ��E����������β������Ӱ�������ʣ�b�������⣻
c.D�ձ��е�ˮ����ȴ���ã�ȥ����������ӷ��Ӿ磬�Ӷ�ʹ���ʽ��ͣ�c�������⣻
d.װ��C�е�NaOH�ɳ�ȥCO2��SO2�����ʣ���Щ����������ȥ���������ˮ��Ӧ���Ͳ��ʣ�ˮ���ն������������Ч����ᵼ�²�����ʽ��ͣ�d�������⣻
e.D���Թ����ˮ��Һ����Ϸ����Ƕ�Һ�����Һ�⣬������Ļӷ�����D�е��Թ��ﲻ��ˮ����ӷ��Ķ࣬����IJ��ʽ��ͣ�e�������⣻
��ˣ�������ȷ���ǣ�b��

 ��ְٷְټ���ϵ�д�
��ְٷְټ���ϵ�д� �����ƻ���ĩ��̶�100��ϵ�д�
�����ƻ���ĩ��̶�100��ϵ�д� �ܿ���ȫ��100��ϵ�д�
�ܿ���ȫ��100��ϵ�д�