��Ŀ����
����Ŀ����ͨ���������̻��շϾ�����ӵ����������(LiCoO2��������Al��Fe)�е��ܺ�ﮡ�
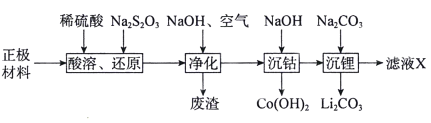
�ش��������⣺
(1)�����ܡ���ԭ��������S2O32-ת��ΪSO42-��LiCoO2���뷴Ӧ�����ӷ���ʽΪ____________________________________��
(2)������������Ҫ�ɷ�Ϊ___________________��
(3)�����ܡ������У�����Һ��pH=10ʱ��c(Co2+)=______mol�� L-1(��֪������KSP[Co(OH)2]=1.58��10-15)��
(4)�ڿ����м���Co(OH)2��������������¶ȵı仯��ͼ��ʾ��

290��ʱ����ȫ��ˮ��ΪCo2O3����Ӧ�Ļ�ѧ����ʽΪ_______________��500��ʱ����Ҫ����Ϊ_____________(�ѧʽ)����1000��ʱ�ķֽ����1mol��2.2mol Na2O(�Թ���)�ڳ�벷�չ��й��ȣ��������ʺ�ɫ�ľ��壬�þ����������Ϊ�������νṹ������Ļ�ѧʽΪ__________________��
(5)����ҺX��������Ҫ��������_______________(�ѧʽ)��
���𰸡�S2O32-+8LiCoO2+22H+=2SO42-+8Li++8Co2++11H2O Al(OH)3��Fe(OH)3 1.58��10-7 4Co(OH)2+O2![]() 2Co2O3+4H2O Co3O4 Na4CoO3 Na2SO4
2Co2O3+4H2O Co3O4 Na4CoO3 Na2SO4
��������
�������������Ҫ����LiCoO2������Al��Fe�ȣ�����ϡH2SO4�ܽ���������ܽ���������������������������Na2S2O3��S2O32-��������SO42-�����ɵ���Һ�к�������ﮡ������ܡ������ƣ���������������Һ��ͨ�����������������Ϊ�����ӣ��γ�������������������������������Һ�м��������������Ƶ�����ҺpH�������ܣ����˵õ��������ܣ���Һ�м���̼���Ƶ�����ҺpH������������γ�̼��ﮣ��õ���ҺX����Ҫ����Ϊ�����ơ�
��1���������Ϻ���LiCoO2������Al��Fe�ȣ�����ϡH2SO4��Na2S2O3��S2O32-��������SO42-�����л�ԭ�ԣ�����������ֻ��LiCoO2���������ԣ���Na2S2O3��Ӧ����CoSO4����Ӧ��ѧ����ʽΪ��8LiCoO2+Na2S2O3+11H2SO4=4Li2SO4+8CoSO4+Na2SO4+11H2O�������ӷ�Ӧ����ʽΪ��S2O32-+8LiCoO2+22H+=2SO42-+8Li++8Co2++11H2O���ʴ�Ϊ��S2O32-+8LiCoO2+22H+=2SO42-+8Li++8Co2++11H2O��
��2����������������Һ��ͨ�����������������Ϊ�����ӣ����γ��������������������ij��������ԡ�����������Ҫ�ɷ�Ϊ��Al(OH)3��Fe(OH)3���ʴ�Ϊ��Al(OH)3��Fe(OH)3��
��3������Һ��pH=10ʱ����Һ��c��H+��=10-10mol/L��c��OH-��=10-4mol/L����Ksp[Co(OH)2]=c��Co2+����c2��OH-��=1.58��10-15��c��Co2+��=1.58��10-7mol��L-1���ʴ�Ϊ��1.58��10-7��
��4�����������غ㶨�ɣ��ڱ仯�����У�Co������û�б䣬����ԭʼ��������Ϊ100g����n��Co��=![]() mol��m��Co��=59��
mol��m��Co��=59��![]() g����1000��ʱ�������������ٱ仯��˵��Co��OH��2��ȫ�ֽ⣬n��Co����n��O��=
g����1000��ʱ�������������ٱ仯��˵��Co��OH��2��ȫ�ֽ⣬n��Co����n��O��=![]() ��[��80.65-59��
��[��80.65-59��![]() ����16]=1��1��ʣ�����ɷ�CoO����500�棬n��Co����n��O��=
����16]=1��1��ʣ�����ɷ�CoO����500�棬n��Co����n��O��=��[��86.38-59��
![]() ����16]=3��4����ѧʽΪCo3O4����290�棬n��Co����n��O��=
����16]=3��4����ѧʽΪCo3O4����290�棬n��Co����n��O��=![]() ��[��89.25-59��
��[��89.25-59��![]() ����16]=2��3����ѧʽΪCo2O3��290��ʱ��Co(OH)2��ȫ��ˮ��ΪCo2O3����Ӧ�Ļ�ѧ����ʽΪ4Co(OH)2+O2
����16]=2��3����ѧʽΪCo2O3��290��ʱ��Co(OH)2��ȫ��ˮ��ΪCo2O3����Ӧ�Ļ�ѧ����ʽΪ4Co(OH)2+O2![]() 2Co2O3+4H2O ��500��ʱ����Ҫ����Ϊ��Co3O4����1000��ʱ�ķֽ����Ϊ��CoO��1molCoO��2.2mol Na2O�ڳ�벷�չ��й��ȣ��������ʺ�ɫ�ľ��壬������Ϊ�������νṹ����������Ϊ��CoO34-���侧��Ļ�ѧʽΪ��Na4CoO3���ʴ�Ϊ��4Co(OH)2+O2
2Co2O3+4H2O ��500��ʱ����Ҫ����Ϊ��Co3O4����1000��ʱ�ķֽ����Ϊ��CoO��1molCoO��2.2mol Na2O�ڳ�벷�չ��й��ȣ��������ʺ�ɫ�ľ��壬������Ϊ�������νṹ����������Ϊ��CoO34-���侧��Ļ�ѧʽΪ��Na4CoO3���ʴ�Ϊ��4Co(OH)2+O2![]() 2Co2O3+4H2O��
2Co2O3+4H2O��
Co3O4��Na4CoO3��
��5���������������õ��ġ���ҺX��������Ҫ�������ǣ�Na2SO4���ʴ�Ϊ��Na2SO4��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��ij�о�С��ͬѧ��̽��ij�����ڷ���һ��ʱ�����Ϊ������������ͥ�վ�Ʒ�������Ļ��ʵ���Ҫ�ɷּ�������ʡ����ȶԸû��ʵijɷֽ��������¼��裺
a��ֻ����FeSO4
b������FeSO4��Fe2(SO4)3
c��ֻ����Fe2(SO4)3
�����ʹ����ĩ����ˮ�еõ���Һ(��ΪX)����������ʵ�飺
ʵ����� | ���� | ���� |
�� | ȡ2 mL��ҺX������1 mL 1 mol��L��1 NaOH��Һ | �������ɫ���� |
�� | ȡ2 mL��ҺX������1��KSCN | ��Һ�Ժ�ɫ |
��1���������ֱ�����������b��������__________________________��
��2����ʵ�颡��Ԥ�������Dz�����ɫ��������Ϊ����ɫ�������ֺ��ɫ������Ԥ�ڲ����������������(�û�ѧ����ʽ�����ӷ���ʽ����)_____��_____��
��3����ʵ�颢�ó��Ľ�����____________�����ʵ�颡�������Ʋ�ʵ�颡ʵ��������Ԥ��������ԭ�������_____________________________��Ϊ��һ����֤���裬С��ͬѧ����������ʵ�飺
ʵ����� | ���� | ���� |
�� | ȡ2 mL��ҺX������1��KSCN���ټ���1 mLˮ | ��Һ�Ժ�ɫ |
�� | ȡ2 mL��ҺX������1��KSCN���ټ���1 mL��ˮ | ��Һ�Ժ�ɫ����ɫ�Ȣ��� |
��4��ʵ�颤����ˮ�μӷ�Ӧ�����ӷ���ʽ��_______________________��
��5��ͨ������ʵ�飬�ɵõ��Ľ�����_____________________________������������ý�������εó���_______________________________��

