��Ŀ����
����Ŀ������ȩ��һ�ֻ���ԭ�ϡ�ijʵ��С����������װ�úϳ�����ȩ�������ķ�Ӧ���£�

CH3CH2CH2CH2OH![]() CH3CH2CH2CHO ����Ӧ��Ͳ������������б����£�
CH3CH2CH2CHO ����Ӧ��Ͳ������������б����£�
�е�/�� | �ܶ�/(g��cm��3) | ˮ���ܽ��� | |
������ | 117.2 | 0.810 9 | �� |
����ȩ | 75.7 | 0.801 7 | �� |
ʵ�鲽�����£���6.0 g Na2Cr2O7����100 mL�ձ��У���30 mLˮ�ܽ⣬��5 mLŨ�����γɻ����Һ����������ҺС��ת����B�С���A�м���4.0 g�������ͼ�����ʯ�����ȡ�������������ʱ����ʼ�μ�B����Һ���μӹ����б��ַ�Ӧ�¶�Ϊ90��95 �棬��E���ռ�90 �����µ���֡�������ﵹ���Һ©���У���ȥˮ�㣬�л������������ռ�75��77 ����֣�����2.0 g��
�ش��������⣺
(1)ʵ���У�Na2Cr2O7��Һ��Ũ�������ӵ�˳��Ϊ___________________________��
(2)�����ʯ��������________________________________________________��
�����Ⱥ���δ�ӷ�ʯ��Ӧ��ȡ����ȷ������______________________________��
(3)����װ��ͼ�У�D������������________��E������������________��
(4)��Һ©��ʹ��ǰ������еIJ�����________��
(5)������ȩ�ֲ�Ʒ���ڷ�Һ©���з�ˮʱ������ȩ��_______________��(��ϡ����¡�)��
(6)��Ӧ�¶�Ӧ������90��95 �棬��ԭ����__________________________________��__________________________________________________��
(7)��ʵ���У�����ȩ�IJ���Ϊ________%�����������λС������
���𰸡���Na2Cr2O7��Һ�еμ�Ũ���� ��ֹ���� ��ȴ�� ������ ��ƿ ��© �� ��֤����ȩ��ʱ���� ������������ȩ����һ������ 51.39��
��������
������Ҫ��������ȩ�Ʊ�ʵ������֪ʶ�����ڿ��鿼����ѧʵ���������������ʵ�������������Aװ��Ϊ��Ӧװ�ý���Һ©���е�Һ����ñ߷�Ӧ��μӵķ�ʽ������Ч���Ʒ�Ӧ���ʣ����ڷ�Ӧ�¶�90��95 �棬Ϊ��ֹ���У���Ҫ�����ʯ�����Ƭ��C1�¶ȼƲ������Ƿ�ӦҺ���¶ȣ� C2�¶ȼƲ��������������¶ȣ�������90 �����£� Eװ�����ռ������Ǵֲ�Ʒ��Ҫ�����ᴿ��������ﵹ���Һ©���У���ȥˮ�㣬�л��㣨��Ҫ������ȩ������������������������ռ�75��77 ����ּ��ɡ�
(1)Ӧ���ܶȴ�ĵ����뵽�ܶ�С��Һ���У������������Һ�ɽ���������Ũ����ϡ�ͣ�
��ȷ�𰸣���Na2Cr2O7��Һ�еμ�Ũ���ᡣ
(2) �ӷ�ʯ�ɷ�ֹҺ�屩�У�������Ǽ����ʯ����Һ��ӽ�����ʱ�����ʯ����Һ���ͻȻ�ͷų�����������������Һ�������ƿ��������Σ�ա������Ҫ���룬���������ȥ��Դ��������Һ����ɼӿ��룻
��ȷ�𰸣���ֹ���� ��ȴ�ӡ�
(3)����������ͼ�ο��ж����������ƣ�D�����������������ܣ�E��������������ƿ��
��ȷ�𰸣������� ��ƿ
(4)��Һ©��ʹ��ǰ�����©��
��ȷ�𰸣���©��
(5) ����ȩ�ܶ�Ϊ0.8017 gcm-3��С��ˮ���ܶȣ�����ˮ�����Ϸ���
��ȷ�𰸣��ϡ�
(6) ������Ŀ������Ӧ��Ͳ���ķе����ݿ�֪����Ӧ�¶ȱ�����90��95�棬�ȿɱ�֤����ȩ��ʱ�������ֿɾ��������䱻��һ��������
��ȷ�𰸣���֤����ȩ��ʱ���� ������������ȩ����һ��������
��7��������ȩ�IJ���Ϊx������������������Ϊx�����ݹ�ϵʽ��
C4H10O �� C4H8O
74 72
4xg2g
��ã�x=74��272��474��272��4=51.39����
��ȷ�𰸣�51��

����Ŀ��ij��ȤС���Է���м�Ƶ���������狀����������Ʊ���ˮ�ϲ�������(FeC2O4��2H2O)����һ���Ʊ��ߴ��Ȼ�ԭ���ۡ�
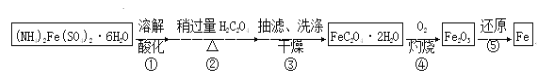
��֪��FeC2O4��2H2O������ˮ��150�濪ʼʧ�ᾧˮ��H2C2O4������ˮ���ܽ�����¶����߶�����
��ش�
��1�����в�����������ȷ����________��
A������ڣ�H2C2O4�Թ�����Ҫ��Ϊ������Fe2+ˮ��
B������ۣ�������ˮϴ�ӿ���߳���Ч��
C������ۣ�ĸҺ�е�������Ҫ��(NH4)2SO4��H2C2O4
D������ۣ�����ڳ�ѹ�¿��ٸ���¶ȿ�ѡ���Ը���100��
��2����ͼװ�ã�����һϵ�в�����ɲ�����еij��˺�ϴ�ӡ���ѡ����ʵı�ţ�����ȷ�IJ���˳������(ϴ�Ӳ���ֻ�迼��һ��)��

�������á�a��b��d��________��c���س����á�
a��ת�ƹ�Һ����b���ػ���A��c��������A��d��ȷ�ϳ�ɣ�e����ϴ�Ӽ�ϴ��
���˺���ͨ������ȣ��ŵ���___________________________________________��
��3�� ��ȡһ������FeC2O4��2H2O�������������ܽ⣬
����KMnO4�ζ����ⶨ�����������£�
n(Fe2+)/mol | n( | ������FeC2O4��2H2O���������� |
9.80��10��4 | 9.80��10��4 | 0.980 |
�ɱ��������Ʋ�����������Ҫ��������____________________��
��4��ʵ�ֲ���ܱ����õ�������������____________________
(��ѡ������a���ձ���b��������c��������ƿ��d������¯��e��������f����ƿ)��
�ò���Ļ�ѧ����ʽ��______________________________________��
��5��Ϊʵ�ֲ���ݣ�������̼�ۻ�ԭFe2O3��������________________________________��
����Ŀ�������仯������NH3����Ρ�N2H4��N2O4������ѧ��ѧ��������ҵ������������ռ����Ҫ��λ��
(1)���亽����������(N2H4)��N2O4��ȼ������ȼ������(N2H4)��N2O4�ķ�ӦΪ
2N2H4 (g)+ N2O4(g)==3N2(g)+4H2O(g) ��H=-1077 kJ��mol-1��
��֪��ط�Ӧ�Ļ�ѧ�������������±���ʾ��
��ѧ�� | N��H | N��N |
| O��H |
E/(kJ��mol��1) | 390 | 190 | 946 | 460 |
��ʹ1 mol N2O4(g)�����л�ѧ����ȫ����ʱ��Ҫ���յ�������________________��
��������˵��2N2H4 (g)+ N2O4(g)==3N2(g)+4H2O(g) ��H ��ƽ��״̬����________
a.��������ƽ����Է����������� b.V��N2��=3V�� N2O4��
c.N2H4���������ֲ��� d. ��H���ٱ仯
(2)N2O4��NO2֮����ڷ�ӦN2O4(g) ![]() 2NO2(g)����һ������N2O4���˺����ܱ������У������ƽ��ת����[��(N2O4)]���¶ȵı仯����ͼ��ʾ��
2NO2(g)����һ������N2O4���˺����ܱ������У������ƽ��ת����[��(N2O4)]���¶ȵı仯����ͼ��ʾ��

����ͼ�Ʋ�÷�Ӧ�ġ�H_______0(��>����<��)������Ϊ____________________________��
��ͼ��a���Ӧ�¶��£���֪N2O4����ʼѹǿp0Ϊ108 kPa������¶��·�Ӧ��ƽ�ⳣ��Kp��________________ (��ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ=��ѹ�����ʵ�������)��
(3)���NO2�Ʊ�NH4NO3���乤��ԭ������ͼ��ʾ��

�������ĵ缫��ӦʽΪ____________________________________________________��
��Ϊʹ������ȫ��ת��ΪNH4NO3���貹��ij�ֻ����������A����A�Ļ�ѧʽΪ________________��