��Ŀ����
16����д�����з�Ӧ�Ļ�ѧ����ʽ����1�������������Ϲ�������һ�ȴ���CH3CH3+Cl2$\stackrel{����}{��}$CH3CH2Cl+HCl��
��2������Ũ����Ļ�Ϲ���C6H6+H2SO4��Ũ���ᣩ$\stackrel{70��-80��}{��}$C6H5SO3H �������ᣩ+H2O��
��3���ɱ�ϩ�ϳɾ۱�ϩ
 ��
����4��������Һ��NaOH��Һ��Ӧ
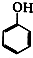 +NaOH��
+NaOH�� +H2O��
+H2O����5����ϩ�������ڼ��������·�����-H ȡ����ӦCH3-CH�TCH2+Cl2$\stackrel{��}{��}$Cl-CH2-CH�TCH2+HCl
��6������ϡ��Һ�еμ���ˮ
 ��
����7����������NaOH���Ҵ��ܹ���CH3CH2Br+NaOH$��_{��}^{�Ҵ�}$CH2�TCH2��+NaBr+H2O��
��8����ȩ��������Һ����������ӦCH3CHO+2Ag��NH3��2OH$\stackrel{ˮԡ}{��}$CH3COONH4+2Ag��+3NH3+H2O��
��9��
 $\stackrel{����KMnO_{4}��Һ}{��}$CO2��
$\stackrel{����KMnO_{4}��Һ}{��}$CO2����10��
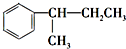 $\stackrel{����KMnO_{4}��Һ}{��}$
$\stackrel{����KMnO_{4}��Һ}{��}$ ��
��
���� ��1���������������Է���ȡ����Ӧ����һ����1����ԭ���滻��1����ԭ�ӣ���һ����ԭ��������ԭ�ӽ�������Ȼ��⣬�ݴ���д��ѧ����ʽ��
��2������Ũ���ᷢ��ȡ����Ӧ���ɱ������ˮ��
��3����ϩ�����Ӿ۷�Ӧ���ɾ۱�ϩ��
��4���������������Ʒ�Ӧ���ɱ����ƺ�ˮ��
��5����ϩ������������-Hȡ�������ϵ�һ���ⱻȡ����
��6����������ˮ��Ӧ���DZ��ӷ������ǻ��Ա���Ӱ�죬�ڶ�λ��ԭ�ӻ��ã�����ȡ����
��7�����������������ƵĴ���Һ���ȷ�����ȥ��Ӧ������ϩ���廯�ƣ�ˮ��
��8����ȩ��������Һ��Ӧ���ɴ���李������ʡ�������ˮ���ݴ�д����Ӧ�Ļ�ѧ����ʽ��
��9����Ȳ����̼̼�����������Ը�����������ɶ�����̼��
��10������ͬϵ������֧���ܱ�����Ӱ�죬����쳣���ã��ܱ����������Һ�����������ᣮ
��� �⣺��1�������������ڹ��յ������·���ȡ����Ӧ����һ����1����ԭ���滻��1����ԭ�ӣ���һ����ԭ��������ԭ�ӽ�������Ȼ��⣬��ѧ��Ӧ����ʽΪ��CH3CH3+Cl2$\stackrel{����}{��}$CH3CH2Cl+HCl��
�ʴ�Ϊ��CH3CH3+Cl2$\stackrel{����}{��}$CH3CH2Cl+HCl��
��2������Ũ���ᷴӦ�������ϵ���ԭ�ӱ������ȡ������ѧ��Ӧ����ʽΪ��C6H6+H2SO4��Ũ���ᣩ$\stackrel{70��-80��}{��}$C6H5SO3H �������ᣩ+H2O��
�ʴ�Ϊ��C6H6+H2SO4��Ũ���ᣩ$\stackrel{70��-80��}{��}$C6H5SO3H �������ᣩ+H2O��
��3����ϩ�ܷ����Ӿ۷�Ӧ�õ��۱�ϩ����ѧ��Ӧ����ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��4�����Ӿ��������ԣ����������Ʒ�Ӧ���ɱ����ƺ�ˮ����ѧ��Ӧ����ʽΪ�� +NaOH��
+NaOH�� +H2O��
+H2O��
�ʴ�Ϊ�� +NaOH��
+NaOH�� +H2O��
+H2O��
��5����ϩ����������ȡ����Ӧ����-H��ȡ������ѧ��Ӧ����ʽΪ��CH3-CH�TCH2+Cl2$\stackrel{��}{��}$Cl-CH2-CH�TCH2+HCl��
�ʴ�Ϊ��CH3-CH�TCH2+Cl2$\stackrel{��}{��}$Cl-CH2-CH�TCH2+HCl��
��6����������ˮ��Ӧ���DZ��ӷ������ǻ��Ա���Ӱ�죬�ڶ�λ��ԭ�ӻ��ã�����ȡ����Ӧ����ѧ��Ӧ����ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��7�����������������ƵĴ���Һ���ȷ�����ȥ��Ӧ������ϩ���廯�ƣ�ˮ����Ӧ�Ļ�ѧ����ʽΪ��CH3CH2Br+NaOH$��_{��}^{�Ҵ�}$CH2�TCH2��+NaBr+H2O��
�ʴ�Ϊ��CH3CH2Br+NaOH$��_{��}^{�Ҵ�}$CH2�TCH2��+NaBr+H2O��
��8����ȩ��������Һ�ķ�Ӧ��ȩ�����л�ԭ�ԣ���������������Ϊ���ᣬͬʱ���ɵ���������Ӧ�Ļ�ѧ����ʽΪ��CH3CHO+2Ag��NH3��2OH$\stackrel{ˮԡ}{��}$CH3COONH4+2Ag��+3NH3+H2O��
�ʴ�Ϊ��CH3CHO+2Ag��NH3��2OH$\stackrel{ˮԡ}{��}$CH3COONH4+2Ag��+3NH3+H2O��
��9����Ȳ����̼̼�����������Ը�����������ɶ�����̼����Ӧ�Ļ�ѧ����ʽΪ��CH��CH$\stackrel{KMnO_{4}}{��}$2CO2��
�ʴ�Ϊ��CO2��
��10��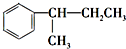 ����������Һ��Ӧ���ɱ����ᣬ��Ӧ�Ļ�ѧ����ʽΪ��
����������Һ��Ӧ���ɱ����ᣬ��Ӧ�Ļ�ѧ����ʽΪ��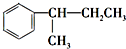 $\stackrel{KMnO_{4}}{��}$
$\stackrel{KMnO_{4}}{��}$ ��
��
�ʴ�Ϊ�� $\stackrel{KMnO_{4}}{��}$
$\stackrel{KMnO_{4}}{��}$ ��
��
���� ���⿼���˷���ʽ����д�����ݷ�Ӧ�������ͷ�Ӧ������д����ʽ����ȷ�����Ļ�ѧ��Ӧԭ���ǽ����Ĺؼ���ע�ⳣ���л�������ʣ���Ŀ�Ѷ��еȣ�

 ����mA��s��+nB��g��?pC��g��+Q�Ŀ��淴Ӧ����һ���¶���B�İٷֺ�����ѹǿ�Ĺ�ϵ��ͼ��ʾ���������ж���ȷ���ǣ�������
����mA��s��+nB��g��?pC��g��+Q�Ŀ��淴Ӧ����һ���¶���B�İٷֺ�����ѹǿ�Ĺ�ϵ��ͼ��ʾ���������ж���ȷ���ǣ�������| A�� | m+n��p | B�� | n��p | ||
| C�� | x���״̬��v����v�� | D�� | ���ϴ𰸶����� |
| A�� | �������Ż�ʱʹ����ĭ�������� | |
| B�� | ���Թܼ���̼�����ƹ���ʱʹ�Թܿ���ֱ���� | |
| C�� | Ũ���ὦ��Ƥ����ʱ������ϡ����������Һ��ϴ | |
| D�� | ������ʵ��ʱ������ڷ���ǰ�������Ǽӷ�ʯ��Ӧ����ֹͣ���ȣ���ȴ�� |
| A�� | K1=16 | B�� | K1=8 | C�� | K2=1 | D�� | K2=0.5 |
| A�� |  �� �� | |
| B�� | CH2�TCH-CH2-CH3+HCl$��_{��}^{����}$ | |
| C�� | CH3-CH�TCH2+H2O$��_{���ȡ���ѹ}^{����}$ | |
| D�� | nCH2�TCH2+nCH2�TCH-CH3$��_{��}^{����}$ |
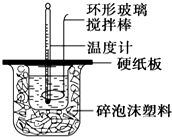 ijʵ��С�������50mL 1.0mol/L�����50mL 1.1mol/L ����������Һ����ͼװ���н����кͷ�Ӧ���ڴ��ձ��ײ�������ĭ���ϣ���ֽ������ʹ�����С�ձ���������ձ�������ƽ��Ȼ�����ڴ�С�ձ�֮����������ĭ���ϣ���ֽ���������ձ�������ĭ���ϰ壨��Ӳֽ�壩���ǰ壬�ڰ��м俪����С�ף�����ʹ�¶ȼƺͻ��β��������ͨ����ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��Իش��������⣺
ijʵ��С�������50mL 1.0mol/L�����50mL 1.1mol/L ����������Һ����ͼװ���н����кͷ�Ӧ���ڴ��ձ��ײ�������ĭ���ϣ���ֽ������ʹ�����С�ձ���������ձ�������ƽ��Ȼ�����ڴ�С�ձ�֮����������ĭ���ϣ���ֽ���������ձ�������ĭ���ϰ壨��Ӳֽ�壩���ǰ壬�ڰ��м俪����С�ף�����ʹ�¶ȼƺͻ��β��������ͨ����ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ��Իش��������⣺��1����ʵ�������Թ�����NaOH��ԭ���DZ�֤������ȫ���кͣ��ڴ�С�ձ��������ĭ���ϵ������DZ��¸��ȣ���ֹ����ɢʧ��
��2����ʵ��С����������ʵ�飬ÿ��ȡ��Һ��50mL������¼��ԭʼ���ݣ����±�����
| ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶ȣ�t2��/�� | �²� ��t2-t1��/�� | ||
| ���� | NaOH��Һ | ƽ��ֵ | |||
| 1 | 25.1 | 24.9 | 25.0 | 31.6 | 6.6 |
| 2 | 25.1 | 25.1 | 25.1 | 31.8 | 6.7 |
| 3 | 25.1 | 25.1 | 25.1 | 31.9 | 6.8 |
��3�����õ�Ũ�ȵĴ�����NaOH��Һ��Ӧ�����õ��к��Ȼ�ȣ�2������ġ�Hƫ���ƫ����ƫС�����䡱������ԭ���Ǵ��������ᣬ��Ӧʱ��Ҫ�����������ڴ���ĵ��룬���ԣ���õ��кͷ�Ӧ�ķ�Ӧ����ֵ��ƫС���÷�ӦΪ���ȷ�Ӧ����H��ƫ��
��4�����к��Ȳⶨʵ���д�����ˮϴ���¶ȼ��ϵ�����������¶ȼƲⶨNaOH��Һ�¶ȵIJ��裬���˲������裬���õ��к��ȡ�H��ƫ���ƫ����ƫС�����䡱����
| A�� | �٢ڢ� | B�� | �ڢܢ� | C�� | �٢ܢ� | D�� | �ڢۢ� |
| A�� | ����Ԫ��ȫ���Ǹ���Ԫ�� | |
| B�� | ���ڱ��е����ڷ�Ϊ�������ڡ������� | |
| C�� | ���ڱ��е����Ϊ�����塢���塢0���VIII�� | |
| D�� | �ɶ�����Ԫ�غͳ�����Ԫ�ع�ͬ��ɵ����Ϊ���壨��0���⣩ |
