��Ŀ����
����8�֣������������壨FeC2O4��2H2O�������������Լ�����Ӱ���ȡ���ͼ�ǽ�һ�������IJ��������������Χ�н������ط�����ʾ��ͼ��TG%��ʾ������������ռԭ��Ʒ�����İٷ���������ش��������⣺
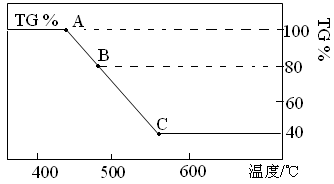
��1��B��������Ļ�ѧʽΪ ��C��������Ļ�ѧʽΪ ��
��2��A��C������Ӧ�������ܷ�Ӧ�Ļ�ѧ����ʽΪ ��
��3������FeC2O4��2H2O����������н������ط�����ԭ���� ��
�����ֽ�õ���600��ʱ�Ĺ�����������Ũ���ᷴӦ����ҺŨ������ȴ���д�9���ᾧˮ�ľ����������þ���Ļ�ѧʽΪ ��
��4����ȡ1.44gFeC2O4����ij��յ��ܱ������У��ٳ���0.04molCO��������1100�棬���з�Ӧ��FeO(s) + CO(g) Fe(s) + CO2(g)��ƽ�ⳣ��K=1/3����Ӧ��ƽ��ʱFeO��ת����Ϊ ��
Fe(s) + CO2(g)��ƽ�ⳣ��K=1/3����Ӧ��ƽ��ʱFeO��ת����Ϊ ��

��1��B��������Ļ�ѧʽΪ ��C��������Ļ�ѧʽΪ ��
��2��A��C������Ӧ�������ܷ�Ӧ�Ļ�ѧ����ʽΪ ��
��3������FeC2O4��2H2O����������н������ط�����ԭ���� ��
�����ֽ�õ���600��ʱ�Ĺ�����������Ũ���ᷴӦ����ҺŨ������ȴ���д�9���ᾧˮ�ľ����������þ���Ļ�ѧʽΪ ��
��4����ȡ1.44gFeC2O4����ij��յ��ܱ������У��ٳ���0.04molCO��������1100�棬���з�Ӧ��FeO(s) + CO(g)
 Fe(s) + CO2(g)��ƽ�ⳣ��K=1/3����Ӧ��ƽ��ʱFeO��ת����Ϊ ��
Fe(s) + CO2(g)��ƽ�ⳣ��K=1/3����Ӧ��ƽ��ʱFeO��ת����Ϊ ����1��Fe2C2O4 FeO ��2�� Fe2C2O4��2H2O FeO+CO��+CO2��+2H2O
��3����ֹFeC2O4�ڼ��ȹ����б������е����������� Fe2(SO4)3��9H2O ��4��50%
��3����ֹFeC2O4�ڼ��ȹ����б������е����������� Fe2(SO4)3��9H2O ��4��50%
A��B��ΪFeC2O4��2H2O����FeC2O4��ˮ�Ĺ��̣�B��60%��ΪFeC2O4����FeCO3��CO���̣�60%��C��ΪFeCO3����FeO��CO2�Ĺ��̣�����
��1��B��������Ļ�ѧʽΪFe2C2O4��C��������Ļ�ѧʽΪFeO��
��2��A��C������Ӧ�������ܷ�Ӧ�Ļ�ѧ����ʽΪ��Fe2C2O4��2H2O==FeO+CO��+CO2��+2H2O��
��3��FeC2O4��2H2O����������н������ط�����ԭ���ǣ���ֹFeC2O4�ڼ��ȹ����б������е�����������
��4���ɿ��淴Ӧ���̡����η�������ʼ���ʵ������仯���ʵ�����ƽ�����ʵ������ɵã���Ӧ��ƽ��ʱFeO��ת����Ϊ50%��
��1��B��������Ļ�ѧʽΪFe2C2O4��C��������Ļ�ѧʽΪFeO��
��2��A��C������Ӧ�������ܷ�Ӧ�Ļ�ѧ����ʽΪ��Fe2C2O4��2H2O==FeO+CO��+CO2��+2H2O��
��3��FeC2O4��2H2O����������н������ط�����ԭ���ǣ���ֹFeC2O4�ڼ��ȹ����б������е�����������
��4���ɿ��淴Ӧ���̡����η�������ʼ���ʵ������仯���ʵ�����ƽ�����ʵ������ɵã���Ӧ��ƽ��ʱFeO��ת����Ϊ50%��

��ϰ��ϵ�д�
 Сѧ�̲�ȫ��ϵ�д�
Сѧ�̲�ȫ��ϵ�д�
�����Ŀ
 ��ij��Һ��(ֻ��Դ��ˮ��������Ӳ�����)��������п��,����ʹ���ַ�Ӧ,����˵������ȷ���ǣ� ��
��ij��Һ��(ֻ��Դ��ˮ��������Ӳ�����)��������п��,����ʹ���ַ�Ӧ,����˵������ȷ���ǣ� ��
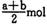 B (a �� b) mol
B (a �� b) mol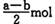 D. (a+b) mol
D. (a+b) mol