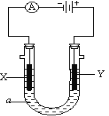
��Ŀ����
����Ŀ��ͨ����ȼ�յķ����ⶨ�л���ĵķ���ʽ������ȼ�����ڽ��л�����Ʒ�봿���ڵ�¯�����³��ȼ�գ����ݲ�Ʒ�ĵ�����ȷ���л������ɡ���ͼ��ʾ������ȼ�շ�ȷ���л��������ʽ�ij���װ�á�
�ش��������⣺
��1���ø÷����ɲⶨ������ЩԪ�غ�ʲô״̬���л���____________________________��
��2��A��B���ھ�ʢ���й�̬�Լ���B�ܵ�������_________________________________��
��3��������������������������ѡ�õĸ����ܿ����ӵ�˳����___________________��
��4��E��Ӧʢװʲô�Լ���_____________________��
��5�������CuO��ȥ����A��������_________�������������С�������䡱��
��6����ȷ��ȡ1.8g��Ʒ��ֻ��C��H��O����Ԫ���е����ֻ����֣��������ȼ�պ�A������1.76g��B������0.36g������л���ĵ����ʽΪ_______________��
��7��Ҫȷ�����л���ķ���ʽ��������֪����������_____________���ɲ��õ��ִ�����������_________________��
��8��������ʵ�鿪ʼ֮ǰ��������D����������ͨ������װ��һ��ʱ�䣬��Ŀ����________��
���𰸡�ֻ��C��H��C��H��OԪ�صĹ�̬�л�������ȼ�պ������ˮ����g��f��e��h��I��c��d����d��c����a��b����H2O2��СCHO2��Ʒ��Ħ�����������ϳ����ڿ�������Сʵ�����
��������
��1������ʹ��ȼ�շ�ȷ���л���ķ���ʽ�����õ������ʽ������Ϊ�ǹ�����Ʒ����ֻ������H2O��CO2��װ�ã����Ը÷���ֻ�ܲⶨ��C��H��C��H��OԪ�صĹ�̬�л��
��ˣ�������ȷ���ǣ�ֻ��C��H��C��H��OԪ�صĹ�̬�л��
��2��B����װ���Ǹ������������������ȼ�պ������ˮ������
��ˣ�������ȷ���ǣ�����ȼ�պ������ˮ������
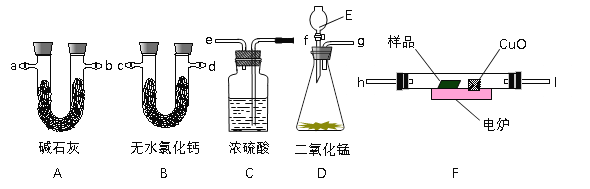
��3��װ�õ����Ӱ�����������������������ȼ�ա�����H2O������CO2����˳����������˳��Ϊ��D��C��F��B��A���ӿ�˳����Ϊg��f��e��h��I��c��d����d��c����a��b����
��ˣ�������ȷ���ǣ�g��f��e��h��I��c��d����d��c����a��b����
��4��Dװ�����Ʊ�O2�ķ���װ�ã�����E��ʢװ����H2O2��
��ˣ�������ȷ���ǣ�H2O2��
��5��F��CuO����������ʹ�л���������������CO2��H2O�����ȥ����H2O������������٣���ôA����������С��
��ˣ�������ȷ���ǣ���С��
��6��ȼ�պ�A������1.76g������CO2��������1.76g�����ʵ�����![]() ��0.04mol��B ������0.36g����������ˮ��0.36g�����ʵ�����
��0.04mol��B ������0.36g����������ˮ��0.36g�����ʵ�����![]() =0.02mol����������������ԭ�ӵ����ʵ�����0.04mol��2��0.02mol��1��0.10mol�����������غ㶨�ɿ�֪���μӷ�Ӧ��������1.76g��0.36g��1.8g��0.32g�����ʵ�����
=0.02mol����������������ԭ�ӵ����ʵ�����0.04mol��2��0.02mol��1��0.10mol�����������غ㶨�ɿ�֪���μӷ�Ӧ��������1.76g��0.36g��1.8g��0.32g�����ʵ�����![]() =0.01mol������л����к�����ԭ�ӣ����ʵ�����0.1mol��0.01mol��2��0.08mol������̼���⡢����ԭ�Ӹ���֮����:0.04:0.02
=0.01mol������л����к�����ԭ�ӣ����ʵ�����0.1mol��0.01mol��2��0.08mol������̼���⡢����ԭ�Ӹ���֮����:0.04:0.02![]() :0.08=1�U1�U2�������ʽΪCHO2��
:0.08=1�U1�U2�������ʽΪCHO2��
��ˣ�������ȷ���ǣ�CHO2��
��7���������ʽ��Ҫ�������ʽ��������֪����Ʒ��Ħ����������������������������
��ˣ�������ȷ���ǣ���Ʒ��Ħ��������������
��8������װ���ں��п������������к���CO2��ˮ���������Ա���ϳ����ڿ�������Сʵ����
��ˣ�������ȷ���ǣ��ϳ����ڿ�������Сʵ����

 �߽�������ϵ�д�
�߽�������ϵ�д�����Ŀ������ΪԪ�����ڱ��е�һ���֣��û�ѧʽ��Ԫ�ط��Żش��������⣺
������ | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
2 | �� | �� | ||||||
3 | �� | �� | �� | �� | �� | |||
4 | �� | �� | �� |
��1��11��Ԫ���У���ѧ��������õ���______��
��2���٢ڢ��У�����������ˮ���������ǿ����________��
��3���ڢۢ����γɵļ����Ӱ뾶�ɴ�С��˳����________��
��4��Ԫ�آ��⻯�ﳣ���º�Ԫ�آڵĵ��ʷ�Ӧ�����ӷ���ʽ��_____________�����⻯����Ԫ�آ�ĵ��ʷ�Ӧ�����ӷ���ʽ��_________________________ ��
��5���ٺ͢������������Ӧ��ˮ���ﻯѧʽΪ________��________���ٺ͢���Ԫ���γɻ�����Ļ�ѧʽΪ________���û���������ʱ��ɫΪ________���û��������Һ��Ԫ�آ�ĵ��ʷ�Ӧ�����ӷ���ʽΪ__________________��
��6���ٺ͢�����������Ӧ��ˮ�������Ӧ�Ļ�ѧ����ʽΪ________��
��7���͢��γɵĻ�����Ļ�ѧʽΪ________���û������ܽ��ĵ���������ҺΪ______________________��
����Ŀ��CoCO3������ѡ�����������αװͿ�ϵ����ϡ��Ժ��ܷ���(��Ҫ��CoO��Co2O3��������Al2O3��ZnO������)Ϊԭ���Ʊ�CoCO3��һ�ֹ�������������
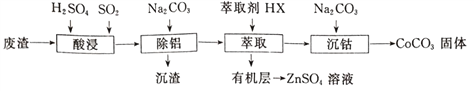
�±�����ؽ������������������������pH(��ʼ������pH����������Ũ��Ϊ1.0mol��L-1����)��
�������� | ��ʼ������pH | ������ȫ��pH |
Co2+ | 7.6 | 9.4 |
Al3+ | 3.0 | 5.0 |
Zn2+ | 5.4 | 8.0 |
��1��д����������ʱ����������ԭ��Ӧ�Ļ�ѧ����ʽ_________________��
��2������������������Ҫ������ҺpH�ķ�ΧΪ______________���γɳ���ʱ������Ӧ�����ӷ���ʽΪ_____________________��
��3����ʵ����������ȡ�����õ��IJ���������Ҫ��____________����������ȡ�����̿ɱ�ʾΪZnSO4(ˮ��)+2HX(�л���) ![]() ZnX2(�л���)+H2SO4(ˮ��)�����л����ȡZnSO4��Һ�IJ�����_________________________��
ZnX2(�л���)+H2SO4(ˮ��)�����л����ȡZnSO4��Һ�IJ�����_________________________��
��4����������ʱ��Na2CO3��Һ�μӹ���ᵼ�²�Ʒ�����������ԭ��_________________��
��5���ڿ���������CoCO3�������������CO2����ó�����պ��������Ϊ2.41g��CO2�����Ϊ0.672L(��״��)�������������Ļ�ѧʽΪ____________________��
����Ŀ������������ʵĵ��볣�����±�:
������� | HCOOH | HCN | H2CO3 |
���볣��(25 ��) | Ka=1.8��10-4 | Ka=4.9��10-10 | Ka1=4.3��10-7 Ka2=5.6��10-11 |
����˵��������ǣ� ��
A. ���H+������:CO32->CN->HCO3->HCOO-
B. 2CN����H2O��CO2=2HCN��CO32-
C. �к͵��������pH��HCOOH��HCN����NaOH����ǰ��С�ں���
D. 25 ��ʱ����ӦHCOOH + CN-![]() HCN+ HCOO-�Ļ�ѧƽ�ⳣ��3.67��105
HCN+ HCOO-�Ļ�ѧƽ�ⳣ��3.67��105