��Ŀ����
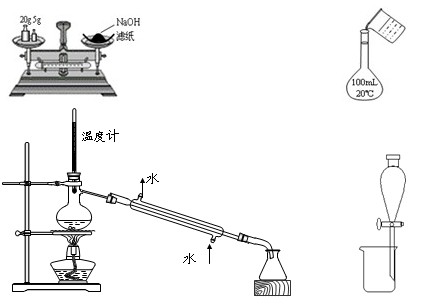
��12�֣���1����4�֣�����ʵ����ƻ������������_________________��
a. �к��Ȳⶨʵ��ʱ�����û�л��β����������û���ͭ�ʽ�������档
b. ʵ�����Ʒ���ʱ��������ֲ���͡��Ҵ���NaOH��Һ��ϣ������Ͻ��衢���ȣ�ֱ��������������ɵõ�������
c. ʵ������ȡ��ϩʱ���뽫�¶ȼƵ�ˮ������뷴ӦҺ�У��ⶨ��ӦҺ���¶ȡ�
d. ��Na2CO3��NaOH�Ļ����Һ�м���������CaCl2��Һ���ٵμӼ��η�̪��Һ���ɼ���NaOH�Ĵ��ڡ�
e. ��10�����������1 mL 10%���ռ���Һ�м���Ƭ�̺��ٵμ�2��2%����������Һ���Լ���ˮ�����ɵ������ӡ�
��2����8�֣���ȡ������������ʵ���Ũ�ȵ������������Һ�ֱ����������������ͬ��п��Ӧ��ͨ������H2�Ŀ���֤�����������ᡣȡ�á���ͬ����п��Ŀ����_____________________________���Ƚ�����H2�����ķ�����_____________
______________________________________��
����������ˮ��ʵ��ʱ��������������NaOH��Һ������������____________________������������ȫˮ���������___________________��
a. �к��Ȳⶨʵ��ʱ�����û�л��β����������û���ͭ�ʽ�������档
b. ʵ�����Ʒ���ʱ��������ֲ���͡��Ҵ���NaOH��Һ��ϣ������Ͻ��衢���ȣ�ֱ��������������ɵõ�������
c. ʵ������ȡ��ϩʱ���뽫�¶ȼƵ�ˮ������뷴ӦҺ�У��ⶨ��ӦҺ���¶ȡ�
d. ��Na2CO3��NaOH�Ļ����Һ�м���������CaCl2��Һ���ٵμӼ��η�̪��Һ���ɼ���NaOH�Ĵ��ڡ�
e. ��10�����������1 mL 10%���ռ���Һ�м���Ƭ�̺��ٵμ�2��2%����������Һ���Լ���ˮ�����ɵ������ӡ�
��2����8�֣���ȡ������������ʵ���Ũ�ȵ������������Һ�ֱ����������������ͬ��п��Ӧ��ͨ������H2�Ŀ���֤�����������ᡣȡ�á���ͬ����п��Ŀ����_____________________________���Ƚ�����H2�����ķ�����_____________
______________________________________��
����������ˮ��ʵ��ʱ��������������NaOH��Һ������������____________________������������ȫˮ���������___________________��

��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
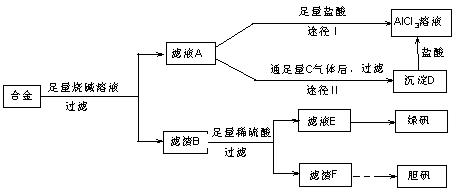
 ��
��
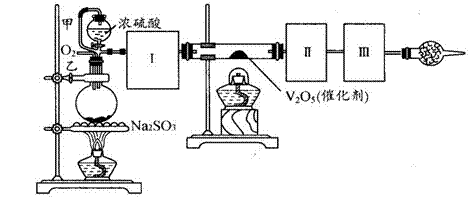
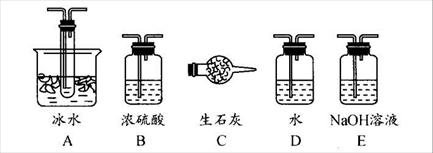
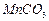 ���Ʊ������ܴ��Բ��ϵ���Ҫԭ�ϡ�ʵ������
���Ʊ������ܴ��Բ��ϵ���Ҫԭ�ϡ�ʵ������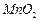 Ϊԭ���Ʊ������ߴ�
Ϊԭ���Ʊ������ߴ�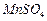 ��Һ��
��Һ��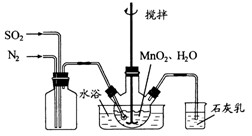
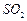 ��
��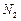 ������壬��Ӧ3h��ֹͣͨ��
������壬��Ӧ3h��ֹͣͨ��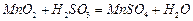 ����
����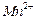 ��
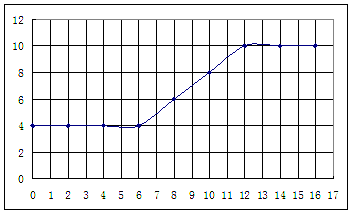
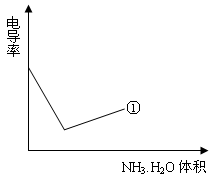
��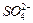 ��Ũ���淴Ӧʱ��t�仯����ͼ��������Һ��
��Ũ���淴Ӧʱ��t�仯����ͼ��������Һ��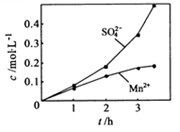
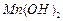 ��ʼ����ʱ
��ʼ����ʱ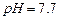 ���벹���ɣ�1���Ƶõ�
���벹���ɣ�1���Ƶõ�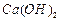 ��
��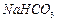 ��
��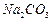 ��
��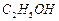 ]��
]��