题目内容
6.现有属于前四周期的A、B、C、D、E、F、G七种元素,原子序数依次增大.A元素的价电子构型为nsnnpn+1;C元素为最活泼的非金属元素,D元素核外有三个电子层,最外层电子数是核外电子总数的1/6;E元素正三价离子的3d轨道为半充满状态,F元素基态原子的M层全充满,N层没有成对电子,只有一个未成对电子;G元素与A元素位于同一主族,其某种氧化物有剧毒.(1)A元素的第一电离能>B元素的第一电离能(填“<”“>”或“=”),A、B、C三种元素电负性由小到大的顺序为N<O<F(用元素符号表示).
(2)D元素原子的价电子排布式是3s2.
(3)C元素的电子排布图为
 ;E3+的离子符号为Fe3+.
;E3+的离子符号为Fe3+.(4)F元素位于元素周期表的ds区,其基态原子的电子排布式为1s22s22p63s23p63d104s1或[Ar]3d104s1.
(5)G元素可能的性质A.
A.其单质可作为半导体材料 B.其电负性大于磷 C.最高价氧化物对应的水化物是强酸.
分析 A元素的价电子构型为nsnnpn+1,则n=2,故A为N元素;C元素为最活泼的非金属元素,则C为F元素;B原子序数介于氮、氟之间,故B为O元素;D元素核外有三个电子层,最外层电子数是核外电子总数的1616,最外层电子数为2,故D为Mg元素;E元素正三价离子的3d轨道为半充满状态,原子核外电子排布为1s22s22p63s23p63d104s2,则原子序数为26,为Fe元素;F元素基态原子的M层全充满,N层没有成对电子,只有一个未成对电子,核外电子排布为1s22s22p63s23p63d104s1,故F为Cu元素;G元素与A元素位于同一主族,其某种氧化物有剧毒,则G为As元素,据此解答.
解答 解:A元素的价电子构型为nsnnpn+1,则n=2,故A为N元素;C元素为最活泼的非金属元素,则C为F元素;B原子序数介于氮、氟之间,故B为O元素;D元素核外有三个电子层,最外层电子数是核外电子总数的16,最外层电子数为2,故D为Mg元素;E元素正三价离子的3d轨道为半充满状态,原子核外电子排布为1s22s22p63s23p63d104s2,则原子序数为26,为Fe元素;F元素基态原子的M层全充满,N层没有成对电子,只有一个未成对电子,核外电子排布为1s22s22p63s23p63d104s1,故F为Cu元素;G元素与A元素位于同一主族,其某种氧化物有剧毒,则G为As元素.
(1)N原子最外层为半充满状态,性质稳定,难以失去电子,第一电离能大于O元素,同周期元素从左到右元素的电负性逐渐增强,故电负性N<O<F,
故答案为:>; N<O<F;
(2)D为Mg元素,最外层为3第三电子层,电子数为2,价电子排布式为3s2,故答案为:3s2;
(3)C为F元素,电子排布图为 ,E3+的离子符号为 Fe3+,
,E3+的离子符号为 Fe3+,
故答案为: ; Fe3+;
; Fe3+;
(4)F为Cu,位于周期表ds区,其基态原子的电子排布式为1s22s22p63s23p63d104s1或[Ar]3d104s1,故答案为:ds; 1s22s22p63s23p63d104s1或[Ar]3d104s1;
(5)G为As元素,与Si位于周期表对角线位置,其单质可作为半导体材料,电负性比P小,其非金属性比P的若,因磷酸为中强酸,则As的最高价氧化物对应的水化物是弱酸,
故答案为:A.
点评 本题考查结构性质与位置关系、核外电子排布规律、电离能、电负性等,难度中等,推断元素是解题的关键,注意基础知识的掌握.

 阅读快车系列答案
阅读快车系列答案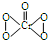 下列说法正确的是( )
下列说法正确的是( )| A. | Cr2O72- 被氧化成CrO5 | |
| B. | 该反应为氧化还原反应 | |
| C. | 反应中H2O2被还原成H2O | |
| D. | 此反应用于检验Cr2O72-离子的存在 |
| A. | 食盐加碘和酱油加铁是为了补存人体所需要的常量元素 | |
| B. | 瘦肉精能提高猪肉的瘦肉率,食用含瘦肉精的猪肉,不会对人体造成危害 | |
| C. | 日本核泄漏事故释放出放射性核素碘-131,极微量碘-131不会对人体产生危害 | |
| D. | 高纯度的硅单质广泛用于制作光导纤维 |
| A. | 向Ba(OH)2溶液中,逐滴加入NaHSO4溶液至Ba2+恰好完全沉淀 | |
| B. | 向NaHSO4溶液中.逐滴加入Ba(OH)2溶液至SO42-恰好完全沉淀 | |
| C. | 向NaHSO4溶液中,逐滴加入Ba(OH)2溶液至过量 | |
| D. | 向pH=1的NaHSO4溶液中加入等体积pH=13的Ba(OH)2溶液 |
①CH4、②CH2=CH2、③HC≡CH、④NH3、⑤NH4+、⑥BF3、⑦H2O.
| A. | 粒子的立体构形呈四面体形的是①⑤ | |
| B. | 中心原子采取sp3杂化的是①④⑤⑦ | |
| C. | 所有原子共线或共面的只有②③⑥ | |
| D. | 粒子中含有配位键的是⑤,含有极性键的极性分子是④⑦ |
| A. | 苯酚钠溶液中通入少量二氧化碳:2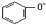 +2H2O+CO2→2 +2H2O+CO2→2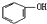 +CO32- +CO32- | |
| B. | 等体积、等浓度的Ca(HCO3)2溶液和NaOH溶液混合:Ca2++2HCO3-+2OH-═CaCO3↓+CO32-+2H2O | |
| C. | 向AlCl3溶液中加入过量氨水:Al3++4NH3•H2O═AlO2-+4NH4++2H2O | |
| D. | 同位素示踪法可用于反应机理的研究:2KMnO4+5H218O2+3H2SO4═K2SO4+2MnSO4+518O2↑+8H2O |
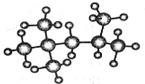 (1)在有机物:①CH3CH3、②CH2=CH2、③CH3CH2C≡CH、④CH3C≡CCH3、
(1)在有机物:①CH3CH3、②CH2=CH2、③CH3CH2C≡CH、④CH3C≡CCH3、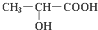 .对于人的身体来说,乳酸是疲劳物质之一,是身体在保持体温和肌体运动而产生热量过程中产生的废弃物.试回答:
.对于人的身体来说,乳酸是疲劳物质之一,是身体在保持体温和肌体运动而产生热量过程中产生的废弃物.试回答: +2Na→
+2Na→ +H2↑,.
+H2↑,.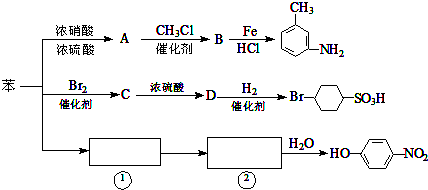
 +CH3Cl
+CH3Cl +HCl.
+HCl. ,②
,② 或
或 .
.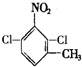 、
、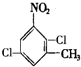
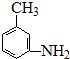 的所有原子不是(填“是”或“不是”)在同一平面上.
的所有原子不是(填“是”或“不是”)在同一平面上.