��Ŀ����
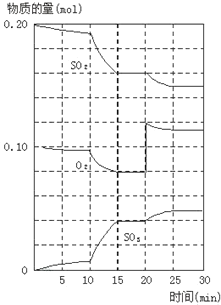
����Ŀ�������ΪVL�ĺ����ܱ�������ʢ��һ����H2��ͨ��Br2(g)������Ӧ��H2(g)��Br2(g)![]() 2HBr(g)����H��0�����¶ȷֱ�ΪT1��T2��ƽ��ʱ��H2�����������Br2(g)�����ʵ����仯��ϵ����ͼ��ʾ������˵������ȷ����(����)
2HBr(g)����H��0�����¶ȷֱ�ΪT1��T2��ƽ��ʱ��H2�����������Br2(g)�����ʵ����仯��ϵ����ͼ��ʾ������˵������ȷ����(����)
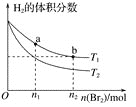
A.��ͼ��֪��T1��T2
B.a��b����ķ�Ӧ���ʣ�b��a
C.Ϊ�����Br2(g)��ת���ʣ��ɲ�ȡ��HBrҺ������ʱ���ߵķ���
D.T1ʱ������Br2(g)���룬ƽ��ʱHBr�����������������
���𰸡�D
��������
A.��ͬͶ���£�������H��0��Ϊ���ȷ�Ӧ��H2�������Խ�ߣ�ת����Խ�ͣ��¶�Խ�ߣ�������ͼ��֪T1��T2����ѡA�
B.��ͬ�¶��£�Ũ��Խ�ߣ���Ӧ����Խ�죬a��b�����¶Ⱦ�ΪT1��b��Br2(g)Ͷ�ϱ�a��࣬��a��b����ķ�Ӧ���ʣ�b��a����ѡB�
C.������Ũ�ȼ�С��ƽ�����ƣ��ʽ�HBrҺ������ʱ���ߣ�Br2(g)ת������ߣ���ѡC�
D. ���¶Ⱦ�ΪT1ʱ������Br2��ƽ���������Ӧ�����ƶ�������HBr�����ʵ��������������������һ�����������������������ת�����йأ���ѡD�
��ѡD��

 �Űٷֿ�ʱ����ϵ�д�
�Űٷֿ�ʱ����ϵ�д�����Ŀ��ѧϰС���о��Ƶ�ȼ�շ�Ӧ���ȡ������(�����ѱ�����)������ú�ͣ����������ڣ��Ϸ�����ʢ��O2����ƿ������һ��ʱ�䣬��ַ�Ӧ��۲쵽����Ϊ��ɫ����͵���ɫ����Ļ�����÷�Ӧǰ������ʵ��������±���
����/g | ||
��Ӧǰ | ���� | 100 |
ʢ�н����Ƶ����� | 105.4 | |
��Ӧ�� | ʢ�й����������� | 107.4 |
��ش��������⣺
(1)����ʵ�������Ʋ�������ɷ�Ϊ___________________���ѧʽ��
(2)������ȫ������ˮ���۲쵽���������ɣ�д���÷�Ӧ�Ļ�ѧ����ʽΪ________��
(3)���������Һ��n(Na+)=0.2mol��ͨ������֤ʵ���ȹ����д��ڱ����Na2Oת��ΪNa2O2��___________________