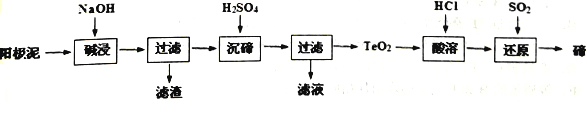
��Ŀ����
����Ŀ���������10��Ԫ�أ�������¸�С�⣮
���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
2 | C | N | O | Ne | ||||
3 | Na | Mg | Al | Si | S | Cl |
��1���ؿ��к�������Ԫ�����������ƣ���
��2������������Ӧ��ˮ���������ǿ�������ѧʽ����
��3��Al��ԭ�ӽṹʾ��ͼΪ ��
��4��S��Cl��ȣ�Ԫ�طǽ����Խ�ǿ���� ��
��5��������������ά�������� �� ��������Ҫ��;����д��һ�֣���
��6����ҵ����N2��H2Ϊԭ�ϣ��ڸ��¡���ѹ�ʹ������ڵ��������Ʊ�NH3 �� ��д����Ӧ�Ļ�ѧ����ʽ�� ��
���𰸡�
��1����������Ԫ�أ�
��2��NaOH
��3��
��4��Cl
��5���������裨��SiO2��,����뵼�����
��6��N2+3H2 ![]() 2NH3
2NH3
���������⣺��1���ؿ��к�������Ԫ������������Ԫ�أ���
���Դ��ǣ���������Ԫ�أ���
��2������������Ӧ��ˮ���������ǿ����NaOH��
���Դ��ǣ�NaOH��
��3��Al��ԭ�ӽṹʾ��ͼΪ 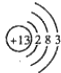 ��
��
���Դ��ǣ�  ��
��
��4��ͬ���ڴ������ҷǽ�������ǿ����S��Cl��ȣ�Ԫ�طǽ����Խ�ǿ����Cl��
���Դ��ǣ�Cl��
��5��������������ά�������Ƕ������裨��SiO2������������Ҫ��;������뵼����ϣ����켯�ɵ�·��̫���ܵ�صȣ���
���Դ��ǣ��������裨��SiO2��������뵼����ϣ�
��6����ҵ����N2��H2Ϊԭ�ϣ��ڸ��¡���ѹ�ʹ������ڵ��������Ʊ�NH3����Ӧ�Ļ�ѧ����ʽΪN2+3H2 ![]() 2NH3�����Դ��ǣ�N2+3H2
2NH3�����Դ��ǣ�N2+3H2 ![]() 2NH3��
2NH3��

 ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�����Ŀ�����и��鷴Ӧ���������ʾ�Ϊ��Ӧ��տ�ʼʱ���ų�H2�����������ǣ� ��
��� | ��������ĩ״�� | ���Ũ�� | ������ | ��Ӧ�¶� |
A | 0.1molMg | 6mol/L���� | 10mL | 60�� |
B | 0.1molMg | 3mol/L���� | 10mL | 60�� |
C | 0.1molFe | 3mol/L���� | 10mL | 60�� |
D | 0.1molMg | 3mol/L���� | 10mL | 30�� |
A.AB.BC.CD.D