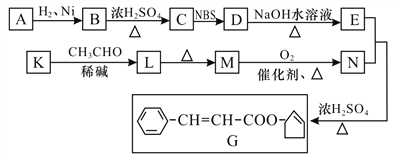
��Ŀ����
����Ŀ��Ϊ��̽�������Ҵ������ʣ�ijͬѧ��Ʋ�����������ʵ�顣
��ش�
(1)����B������Ϊ_____________��
(2)Ϊ�����Ʊ�������A��Ӧ������Լ�Ϊ______��B��Ӧ������Լ�Ϊ_______��
(3)��A�м����Ҵ���B�м�����ʯ�ң�C�м�����ˮ����ͭ����Ӧһ��ʱ���C�е�����Ϊ_________������ⷴӦ�м���������ȩ�����������������ᡣ��д���Ҵ�������ͭ��Ӧ������ȩ�Ļ�ѧ����ʽ_____________________��
(4)��(3)��ʵ���ijͬѧΪ����β�����Ƿ����Ҵ������߿���ѡ���װ����______(��ס����ҡ�)��ʵ��ʱӦ�Ƚ�����״ͭ˿���ȣ���ں��ٳ���Ѹ�������Թ��У��۲쵽ͭ˿�ɺڽ�����죬�ɴ˿ɵó��Ľ�����____________________��(������������)
���𰸡� (����)����� Ũ��ˮ(��ӡ���ʯ�ҡ�ǿ��ȡ����۷�) ��ʯ�� �����ɰ�ɫ��Ϊ��ɫ CH3CH2OH+CuO![]() CH3CHO+Cu+H2O �� ����ȷ��β�����Ƿ����Ҵ�����Ϊ��ȩҲ�ܽ�����ͭ��ԭΪͭ(����������)
CH3CHO+Cu+H2O �� ����ȷ��β�����Ƿ����Ҵ�����Ϊ��ȩҲ�ܽ�����ͭ��ԭΪͭ(����������)
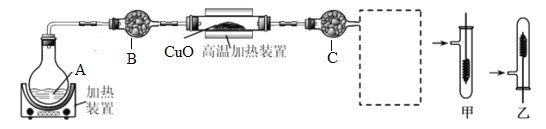
����������1���������������ж��������ƣ�
��2������װ��A�Ʊ��������������ݰ����Ǽ�������ѡ��������
��3�������ڼ��ȵ��������Ҵ��ܱ�����ͭ����Ϊ��ȩ������
��4�������Ҵ��ܱ����ȵ�����ͭ����������ʵ�״̬������������ȩҲ�ܱ�����������
��1�����������Ľṹ��֪����B������Ϊ����ܡ�
��2����ˮ�е�һˮ�ϰ������ֽ����ɰ�������Ϊ�����Ʊ�������A��Ӧ������Լ�ΪŨ��ˮ��Bװ���������ﰱ��������Ӧ������Լ�Ϊ��ʯ�ҡ�
��3����A�м����Ҵ���B�м�����ʯ�ң�C�м�����ˮ����ͭ����Ӧһ��ʱ����Ҵ�������ͭ����Ϊ��ȩ��ͬʱ����ͭ��ˮ�������ɣ���C�е�����Ϊ�����ɰ�ɫ��Ϊ��ɫ���Ҵ�������ͭ��Ӧ������ȩ�Ļ�ѧ����ʽΪCH3CH2OH+CuO![]() CH3CHO+Cu+H2O��
CH3CHO+Cu+H2O��
��4�������Ҵ��ܰ�����ͭ����Ϊ��ȩ������ͭ����ԭΪ��ɫ��ͭ�����Ҵ���Һ�壬�������߿���ѡ���װ���Ǽ�װ�ã�����Ϊ��ȩҲ�ܽ�����ͭ��ԭΪͭ�����Բ�������ͭ˿�ɺڽ������ȷ��β�����Ƿ����Ҵ���

����Ŀ���̵���;�dz��㷺����̼���̿�(��Ҫ�ɷ�ΪMnCO3�����������������ܵ�̼���μ�SiO2����)Ϊԭ�����������̵Ĺ����������£�

��֪25�棬�������ʵ��ܶȻ��������£�
���� | Mn(OH)2 | Co(OH)2 | Ni(OH)2 | Fe(OH)3 | MnS | CoS | NiS |
Ksp | 2.1��10-13 | 3.0��10-16 | 5.0��10-16 | 1.1��10-36 | 1.0��10-11 | 5.0��10-22 | 1.0��10-22 |
��1��������У�Ϊ�ӿ��ܽ����ʣ��ɲ�ȡ�Ĵ�ʩ��____________________�����ٴ�������
��2��������У��Ӱ�ˮ������Һ��pHΪ5.0��6.0��������1�ijɷ���ҪΪ______���ѧʽ������֪MnO2������Ϊ��������������1�����漰�����ӷ���ʽΪ��NH3�qH2O+H+==NH4+ +H2O��__________________��___________________��
��3��������У�����(NH4)2S��Ũ�Ȳ��˹����ԭ����_______________________��
��4����Һ2�У�c(Co2+) ��c(Ni2+)=_______________��
��5��������Ϊa�K��̼���̿��������̴�����õ�����Mn b kg����ÿһ����������ȫ����������1Ϊ����Ԫ�صĴ��������Ϊc kg����ԭ̼���̿���MnCO3����������Ϊ_________���ú�a��b��c��ʽ�ӱ�����軯��