��Ŀ����
����Ŀ����X��Y��Z��W���ֺ�14�����ӵ����ӣ���ṹ�ص����£�
���Ӵ��� | X | Y | Z | W |
ԭ�Ӻ��� | ���� | ��ͬԪ�ع��ɵ����� | ͬԪ�ع��ɵ����� | ͬԪ�ع��ɵ����� |
���ӵĵ���� | 0 | 0 | ��������� | 0 |
![]() ԭ�Ӻ����Xԭ�Ӷ�3�����ӣ�A��ԭ�ӽṹʾ��ͼ�� ______ ��
ԭ�Ӻ����Xԭ�Ӷ�3�����ӣ�A��ԭ�ӽṹʾ��ͼ�� ______ ��![]() �������ᄃ���к��й��ۼ���ĿΪ ______
�������ᄃ���к��й��ۼ���ĿΪ ______
![]() ���������ɵĻ�����ĵ���ʽΪ ______
���������ɵĻ�����ĵ���ʽΪ ______
![]() ��ȫȼ�շų���������
��ȫȼ�շų���������![]() ��д��Yȼ�յ��Ȼ�ѧ����ʽ ______
��д��Yȼ�յ��Ȼ�ѧ����ʽ ______
![]() ���W��Ԫ������������Ӧ��ˮ���������ͼ��ʾת����ϵ
���W��Ԫ������������Ӧ��ˮ���������ͼ��ʾת����ϵ![]() ��Ӧ������������������
��Ӧ������������������![]()
![]()
![]() д�����ڸ�������ˮ��Ӧ�Ļ�ѧ����ʽ ______
д�����ڸ�������ˮ��Ӧ�Ļ�ѧ����ʽ ______
![]() ���W��Ԫ�صļ��⻯�K������ˮ����Ҫԭ���� ______ �����⻯����������Թ���һ��ȼ�ϵ�أ��������Һ��KOH���为���ĵ缫��ӦʽΪ ______ ��
���W��Ԫ�صļ��⻯�K������ˮ����Ҫԭ���� ______ �����⻯����������Թ���һ��ȼ�ϵ�أ��������Һ��KOH���为���ĵ缫��ӦʽΪ ______ ��
���𰸡�![]()
![]()
![]()
![]() 3Fe+4H2O
3Fe+4H2O![]() Fe3O4+4H2��C+H2O
Fe3O4+4H2��C+H2O![]() CO+H2
CO+H2 ![]() ��
��![]() ��������
�������� ![]()
��������
X��Y��Z��W���ֺ�14�����ӵ����ӣ�XΪ���ˣ������Ϊ0����X��������Ϊ14��XΪSiԭ�ӣ�YΪ��ͬԪ�ع��ɵ��������ӣ����ӵ����Ϊ0����YΪCO��ZΪͬԪ�ع��ɵ��������ӣ����ӵ����Ϊ![]() �����γ�ZԪ�ص�ԭ��������Ϊ
�����γ�ZԪ�ص�ԭ��������Ϊ![]() ����ZΪ
����ZΪ![]() ��WΪͬԪ�ع��ɵ��������ӣ����ӵ����Ϊ0�����γ�WԪ�ص�ԭ��������Ϊ
��WΪͬԪ�ع��ɵ��������ӣ����ӵ����Ϊ0�����γ�WԪ�ص�ԭ��������Ϊ![]() ��ΪNԪ�أ�WΪ
��ΪNԪ�أ�WΪ![]() �������Ϸ������
�������Ϸ������
![]() ԭ�Ӻ����Siԭ�Ӷ�3�����ӣ�AΪClԭ�ӣ�ԭ�Ӻ��������Ϊ17����3�����Ӳ㣬���������Ϊ2��8��7��ԭ�ӽṹʾ��ͼΪ
ԭ�Ӻ����Siԭ�Ӷ�3�����ӣ�AΪClԭ�ӣ�ԭ�Ӻ��������Ϊ17����3�����Ӳ㣬���������Ϊ2��8��7��ԭ�ӽṹʾ��ͼΪ![]() ��
��![]() ������ÿ��Siԭ����4��Oԭ��֮���γ�4��
������ÿ��Siԭ����4��Oԭ��֮���γ�4��![]() ����
����![]() �������
�����к���![]() �����ۼ����ʴ�Ϊ��
�����ۼ����ʴ�Ϊ��![]() ��
��![]() ��
��
![]() ̼����Ϊ���ӻ�����ɸ�������
̼����Ϊ���ӻ�����ɸ�������![]() ���ӹ��ɣ�
���ӹ��ɣ�![]() ������Cԭ��֮���γ�3�Թ��õ��Ӷԣ�̼���Ƶ���ʽΪ��
������Cԭ��֮���γ�3�Թ��õ��Ӷԣ�̼���Ƶ���ʽΪ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
![]() �����������ʵ���Ϊ
�����������ʵ���Ϊ![]() ����2molCOȼ�շų�������Ϊ
����2molCOȼ�շų�������Ϊ![]() ��COȼ�յ��Ȼ�ѧ����ʽΪ��
��COȼ�յ��Ȼ�ѧ����ʽΪ��![]()
![]() ���W��Ԫ������������Ӧ��ˮ�����Ϊ
���W��Ԫ������������Ӧ��ˮ�����Ϊ![]() ����ת����ϵ��֪��ΪFe��C����ת����ϵ������ΪFe������Ϊ����������Ϊ��������������ΪC������Ϊ������̼����Ϊһ����̼���ʶ��ڸ�������ˮ��Ӧ�Ļ�ѧ����ʽΪ��3Fe+4H2O
����ת����ϵ��֪��ΪFe��C����ת����ϵ������ΪFe������Ϊ����������Ϊ��������������ΪC������Ϊ������̼����Ϊһ����̼���ʶ��ڸ�������ˮ��Ӧ�Ļ�ѧ����ʽΪ��3Fe+4H2O![]() Fe3O4+4H2��C+H2O
Fe3O4+4H2��C+H2O![]() CO+H2��
CO+H2��
�ʴ�Ϊ��3Fe+4H2O![]() Fe3O4+4H2��C+H2O
Fe3O4+4H2��C+H2O![]() CO+H2��
CO+H2��
![]() ���W��Ԫ�صļ��⻯��Ϊ
���W��Ԫ�صļ��⻯��Ϊ![]() ��
��![]() ��
��![]() ����������
�����γ������![]() ��������ˮ��
��������ˮ��![]() ��������Թ���һ��ȼ�ϵ�أ���������������Ӧ��
��������Թ���һ��ȼ�ϵ�أ���������������Ӧ��![]() �ڸ����ŵ磬��������������
�ڸ����ŵ磬��������������![]() ��
��![]() ����ⷴӦʽΪ��
����ⷴӦʽΪ��![]() ��
��

 �������¿��ÿ�ʱ��ҵϵ�д�
�������¿��ÿ�ʱ��ҵϵ�д� Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�����Ŀ���״�����ȼ������Ҫ�Ļ���ԭ�ϣ��ֿ���Ϊȼ�ϣ����úϳ���![]() ��Ҫ�ɷ�ΪCO��
��Ҫ�ɷ�ΪCO��![]() ��
��![]() �ڴ����������ºϳɼ״������飮��֪�ϳɼ״�����������Ӧ���£���֪CO�ĽṹʽΪC=O������
�ڴ����������ºϳɼ״������飮��֪�ϳɼ״�����������Ӧ���£���֪CO�ĽṹʽΪC=O������![]() ����
����![]() ����
����![]() ��
��
�ش��������⣺
��1����֪��Ӧ������صĻ�ѧ�����������£�
��ѧ�� |
|
|
|
|
|
| 436 | 343 | 1076 | 465 | 413 |
�ɴ˼���![]() ______
______ ![]() ��֪
��֪![]() ����
����![]() ______��
______��
��2�����ݻ�Ϊ![]() �������У�ͨ��һ�����ļ״�������Ӧ��
�������У�ͨ��һ�����ļ״�������Ӧ��![]() ʱ����ϵ�и�����Ũ����ʱ��仯��ͼ��ʾ��ƽ��ʱ�״���ת���ʼ���a1����
ʱ����ϵ�и�����Ũ����ʱ��仯��ͼ��ʾ��ƽ��ʱ�״���ת���ʼ���a1����

����![]() ʱ�Σ���Ӧ����
ʱ�Σ���Ӧ����![]() Ϊ ______ ���÷�Ӧ��ƽ�ⳣ��
Ϊ ______ ���÷�Ӧ��ƽ�ⳣ��![]() �ļ���ʽΪ ______ ��
�ļ���ʽΪ ______ ��
����֪���ں�ѹ�����½��У�ƽ��ʱ![]() ��ת����
��ת����![]() ______
______ ![]() ����������������������������
����������������������������![]() ��������� ______ ��
��������� ______ ��
��3���ϳ�![]() ��ԭ����
��ԭ����![]() ����������ͬ��ʵ������
����������ͬ��ʵ������![]() ��
��![]() ��
��![]() ��
��![]() �����¸÷�Ӧ��
�����¸÷�Ӧ��![]() ƽ��ת������ͬ����
ƽ��ת������ͬ����![]() ����P1______ P2������
����P1______ P2������![]() ������
������![]() ������
������![]() ������
������
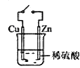
��4����ѧ���õ����ز�����ͭ��װ��ͼ��ʾ���˹����ϵͳ�����ø�װ�óɹ���ʵ������![]() ��
��![]() �ϳ�
�ϳ�![]() ��
��

��д��ͭ�缫����ĵ缫��Ӧʽ ______��
��Ϊ��߸��˹����ϵͳ�Ĺ���Ч�ʣ�����װ���м������� ______ ![]() ѡ��������������������
ѡ��������������������![]() ��
��
��5����״���£���![]() �ļ�����ȫȼ�����ɵ�
�ļ�����ȫȼ�����ɵ�![]() ͨ�뵽
ͨ�뵽![]() ��NaOH��Һ�У�������Һ������Ũ���ɴ�С��˳��Ϊ ______��
��NaOH��Һ�У�������Һ������Ũ���ɴ�С��˳��Ϊ ______��
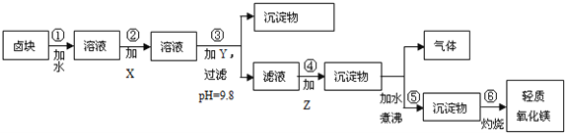
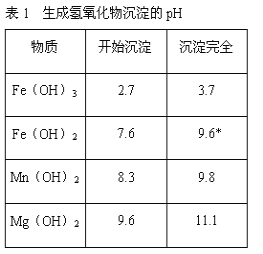
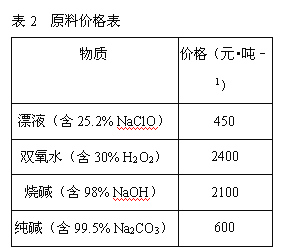
����Ŀ������ʯ����Կ�������MgO��![]() ��
��![]() ��
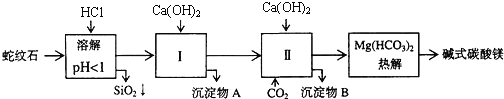
��![]() ��ɣ�������ʯ��ȡ��ʽ̼��þ��ʵ�鲽�����£�
��ɣ�������ʯ��ȡ��ʽ̼��þ��ʵ�鲽�����£�
�������� |
|
|
|
��ʼ����pH |
|
|
|
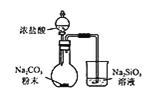
(1)����ʯ��������ܽ����Һ�����![]() �⣬�����еĽ��������� ______
�⣬�����еĽ��������� ______
(2)���Т����ʱ��������Һ![]() �й��������������pH���ϱ�
�й��������������pH���ϱ�![]() ���ܹ�������
���ܹ�������![]() �������ܻᵼ�� ______ �ܽ⡢ ______ �������ɣ�
�������ܻᵼ�� ______ �ܽ⡢ ______ �������ɣ�
(3)�ӳ��������A����ȡ��ɫ�����������ϣ����������A�м��� ______ ![]() �������ʵĻ�ѧʽ
�������ʵĻ�ѧʽ![]() ��Ȼ�� ______
��Ȼ�� ______ ![]() ������дʵ���������
������дʵ���������![]() ��
��
(4)����ѭ��ʹ�ã��ܽ�Լ��Դ������ʵ���У�����ѭ��ʹ�õ������� ______ ![]() ��д���ʻ�ѧʽ
��д���ʻ�ѧʽ![]() ��
��
(5)���ȷֽⲻ��ȫ�����ü�ʽ̼��þ�н�����![]() �����Ʒ��þ���������� ______
�����Ʒ��þ���������� ______ ![]() ����������������������������������֪̼��þ����Է�������Ϊ84����ʽ̼��þ����Է�����������
����������������������������������֪̼��þ����Է�������Ϊ84����ʽ̼��þ����Է�����������![]() ��
��
����Ŀ�����и���ʵ������ó��Ľ�����ȷ����![]()
ѡ�� | ʵ����� | ���� | ���� |
A | �� | �õ�������Һ | X��һ������ |
B | Ũ�Ⱦ�Ϊ | ������ɫ���� |
|
C |
| ��ֽ��Ϊ��ɫ |
|
D |
| �л���ʳ�ɫ | �����ԣ� |
A.AB.BC.CD.D