��Ŀ����
8��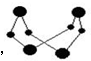 H��C��O��N��S��Cl�dz����ļ��ַǽ���Ԫ�أ�
H��C��O��N��S��Cl�dz����ļ��ַǽ���Ԫ�أ���1��OԪ����Ԫ�����ڱ��е�λ��Ϊ�ڶ����ڵڢ�A�壬OԪ�ص�һ��������Ϊ10��ͬλ�ص�ԭ�ӷ���Ϊ188O��O2-�����ӽṹʾ��ͼΪ
 ��
����2��H2S�ķе��H2O�ͣ�����Ϊˮ���Ӽ���������
��3��ClԪ�صķǽ�����ǿ��SԪ�أ���ԭ�ӽṹ����ԭ��ͬ����Ԫ������ԭ�������ĵ�����ԭ�Ӱ뾶�ݼ����õ���������ǿ���ǽ�������ǿ��
��4����CS2���������ữ�ĸ������ˮ��Һ����Һ��ɫ��ȥ��������ƣ�ͬʱ�ų�CO2��д���÷�Ӧ�Ļ�ѧʽ����ʽ5CS2+4KMnO4+6H2SO4=2K2SO4+4MnSO4+10S��+5CO2��+6H2O��
��5��S4N4�Ľṹ��ͼ���䲻����ˮ�����ڱ�������̼���ܼ��� S4N4�ľ��������Ƿ��Ӿ��壮�ø���İ�������S2Cl2��CCl4��Һ�п���S4N4����ѧ��Ӧ����Ϊ6S2Cl2+16NH3�TS4N4+S8+12NH4Cl��������Ӧ�����У��ƻ����γɵ����������������Ӽ������Լ����Ǽ��Լ�����λ�������»�������д���� ����
��6��A��SO2���ڵȵ����壨��������ͬ��ԭ����������ͬ����������������������������A��һ����ɫ�����壬����ȴ��77Kʱ����ɳȺ�ɫҺ�壮A��һ���Ʊ������ǣ�������У���CuO����һ���ϼ��ȣ�������A��һ��ԭ�Ӹ���֮��Ϊ1��1�ĺ�ɫ����B��д�������Ʊ�A�Ļ�ѧ��Ӧ����ʽCuO+3S$\frac{\underline{\;\;��\;\;}}{\;}$S2O��+CuS��
���� ��1����ԭ����2�����Ӳ㣬�������6�����ӣ������������ڵ��Ӳ������������������������ӣ�OԪ�ص�һ��������Ϊ10��ͬλ�أ�����������Ϊ8��������Ϊ10�������Ӻ�����8�����ӣ�������2�����Ӳ㣬�ֱ�����2��8�����ӣ�
��2��ˮ�����д������������Ĵ����ܹ�ʹ���ʵ��۷е����ߣ�
��3��ͬ����Ԫ������ԭ�������ĵ�����ԭ�Ӱ뾶�ݼ����õ���������ǿ���ǽ�������ǿ��
��4������̼�������������Ի����·���������ԭ��Ӧ���������̡�������̼��ˮ��
��5������Ĺ�����ȷ���������ͣ��ɷ��ӹ��ɵľ���Ϊ���Ӿ��壬��ԭ�ӹ��ɵľ���Ϊԭ�Ӿ��壻�÷�Ӧ6S2Cl2+16NH3=S4N4+S8+12NH4Cl��NH3�к��м��Լ��ͷ��»������Ȼ���к�����λ�������Լ������Ӽ���S8�к��з��»����ͷǼ��Լ���S4N4�к��з��»����ͼ��Լ���
��6��A��SO2���ڵȵ����壬��������һ����ɫ�����壬����ȴ��77Kʱ����ɳȺ�ɫҺ�壬��AΪS2O������Ԫ���غ�͵��ӵ�ʧ�غ��д����ȡA�Ļ�ѧ����ʽ��
��� �⣺��1����ԭ����2�����Ӳ㣬�������6�����������������ڱ���λ�ã��ڶ����ڵڢ�A �壻������Ϊ8��������Ϊ10��ԭ�ӷ���Ϊ��188O�� �����Ӻ�����8�����ӣ�������2�����Ӳ㣬�ֱ�����2��8�����ӣ����ӽṹʾ��ͼ�� ��
��
�ʴ�Ϊ���ڶ����ڵڢ�A �壻 188O�� ��
��
��2��ˮ�����д������������Ĵ����ܹ�ʹ���ʵ��۷е����ߣ�
�ʴ�Ϊ��ˮ���Ӽ���������
��3��ClԪ�غ�SԪ��λ��ͬ���ڣ�ͬ����Ԫ������ԭ�������ĵ�����ԭ�Ӱ뾶�ݼ����õ���������ǿ���ǽ�������ǿ��
�ʴ�Ϊ��ԭ�Ӱ뾶�ݼ����õ���������ǿ��
��4������̼�������������Ի����·���������ԭ��Ӧ���������̡�������̼��ˮ����ѧ����ʽ��5CS2+4KMnO4+6H2SO4=2K2SO4+4MnSO4+10S��+5CO2��+6H2O��
�ʴ�Ϊ��5CS2+4KMnO4+6H2SO4=2K2SO4+4MnSO4+10S��+5CO2��+6H2O����
��5��S4N4�ľ������ɷ��ӹ��ɵģ����ڷ��Ӿ��壬�÷�Ӧ6S2Cl2+16NH3=S4N4+S8+12NH4Cl��NH3�к��м��Լ��ͷ��»������Ȼ���к�����λ�������Լ������Ӽ���S8�к��з��»����ͷǼ��Լ���S4N4�к��з��»����ͼ��Լ��������ƻ����������У����Ӽ������Լ����Ǽ��Լ�����λ�������»�����
�ʴ�Ϊ�����Ӿ��壻���Ӽ������Լ����Ǽ��Լ�����λ�������»�����
��6��A��SO2���ڵȵ����壬��������һ����ɫ�����壬����ȴ��77Kʱ����ɳȺ�ɫҺ�壬��AΪS2O��
��1������Ԫ���غ�͵��ӵ�ʧ�غ��֪��CuO��S��Ӧ����S2O��ͬʱ����һ�ֺ�ɫ����BӦΪCuS����Ӧ�Ļ�ѧ����ʽΪCuO+3S$\frac{\underline{\;\;��\;\;}}{\;}$S2O+CuS��
�ʴ�Ϊ��CuO+3S$\frac{\underline{\;\;��\;\;}}{\;}$S2O+CuS��
���� ���⿼���˻�ѧ�����ʹ�á�����ʽ����д����ѧ�����͵��жϡ�������������ʵ�Ӱ�죬��Ϥ���֪ʶ�ǽ���ؼ�����Ŀ�ѶȽϴ�

 ����ν����Ž̲��㽭���̴�ѧ������ϵ�д�
����ν����Ž̲��㽭���̴�ѧ������ϵ�д� �����Ļ������������������ϵ�д�
�����Ļ������������������ϵ�д�| A�� | ��SO2ͨ��BaCl2��Һ�������ͣ�������������ͨ�����NH3���������� | |
| B�� | �ߴ��賣�������ά��ԭ�� | |
| C�� | ��AlCl3��Һ��ȡAl��OH��3��������ѡ�ð�ˮ��ѡ��NaOH��Һ�� | |
| D�� | ��п��ϡ���ᷴӦ�������������ʽ������ټ�������CuSO4���壬��Ӧ���ʼӿ� |
| A�� | 0.1mol•L-1�ģ�NH4��2SO4��Һ�У�c��SO42-����c��NH4+����c��H+����c��OH-�� | |
| B�� | ��Ũ�ȵ������NaHSO3��Һ��NaClO��Һ��Ϻ�c��Na++c��H+���Tc��HSO3-��+c��ClO-��+2c��SO32-��+c��OH-�� | |
| C�� | ��Ũ�ȵ������NaHCO3��Һ��NaCl��Һ��Ϻ�$\frac{1}{2}$ c��Na+���Tc��HCO3-��+c��CO32-��+c��H2CO3�� | |
| D�� | ����£���2.24LSO2����ͨ�뵽100ml 1mol•L-1��NaOH��Һ�У���ȫ��Ӧ����Һ�����ԣ������Һ�У� c��Na+����c��HSO3-����c��SO32-����c��H+����c��OH-�� |
| A�� | ��ˮ100���25���pHֵ | |
| B�� | Na2CO3��Һ��HCO3-��OH-��Ŀ | |
| C�� | 100mL0.1mol/L��CH3COOH��Һ��10mL1.0mol/L��CH3COOH��Һ��H+��Ŀ | |
| D�� | ͬ����pH=11��KOH��pH=3��CH3COOH��Һ����ˮ�������OH-����Ũ�� |
| A�� | ���ǰ뵼����ϣ���ͬ�����Ҳ�ǰ뵼����� | |
| B�� | Ũ������Ը���HCl���壬��Ҳ����Ũ�������HI���� | |
| C�� | Na�ڿ�����ȼ�ջ�����Na2O2����Li�ڿ�����ȼ��Ҳ������Li2O2 | |
| D�� | ±��Ԫ�ص�����˵���������۵����ߣ��ʼ���������۵�Ҳ��˵�������Ӷ����� |
| A�� | ������ | B�� | ����������Һ | C�� | ���� | D�� | ��ˮ |
| A�� | Ѫ����7.35��7.45�� | B�� | ��Һ��7.5��8.0�� | C�� | θҺ��0.9��1.5�� | D�� | ��Һ��6.6��7.1�� |
�ټӳɢ��������������������û��Ӿۣ�
| A�� | �������� | B�� | ֻ�Т٢ۢ� | C�� | ֻ�Т٢ܢݢ� | D�� | ȫ�� |
| A�� | ��CN��2�ڼ���Һ������CN-��OCN- | |
| B�� | IBr+2NaOH�TNaBr+NaIO+H2O | |
| C�� | �����ڿ�����ȼ�� | |
| D�� | IBr�뱽����ȡ����Ӧ�����屽�͵⻯�� |