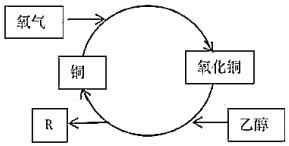
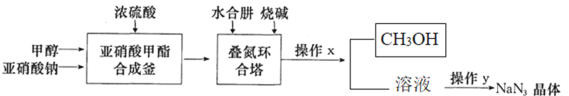
��Ŀ����
����Ŀ��I.�±���Ԫ�����ڱ���һ���֡��������е���ĸ�ֱ����һ�ֻ�ѧԪ�ء�
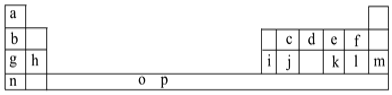
�Իش��������⣺
��1��Ԫ��pΪ26��Ԫ�أ���д�����̬ԭ�ӵ����Ų�ʽ��___��
��2��d��a��Ӧ�IJ���ķ���������ԭ�ӵ��ӻ���ʽΪ___���÷�����___(����ԡ��Ǽ��ԡ�)���ӡ�
��3��h�ĵ����ڿ�����ȼ�շ���ҫ�۵İ⣬����ԭ�ӽṹ��֪ʶ���ͷ����ԭ��__��
��4��o��p��Ԫ�صIJ��ֵ��������������±���
Ԫ�� | o | p | |
������/kJ��mol��1 | I1 | 717 | 759 |
I2 | 1509 | 1561 | |
I3 | 3248 | 2957 | |
�Ƚ���Ԫ�ص�I2��I3��֪����̬o2����ʧȥһ�����ӱ���̬p2����ʧȥһ�������ѡ��Դˣ���Ľ�����___��
II.��1����̬��ԭ���У�����ռ������ܼ����ӵĵ���������ͼ��״Ϊ__�����һ��ͬ�����������ʽΪS8����ṹ��ͼ��ʾ������Sԭ�ӵ��ӻ��������Ϊ___��S8�����ڶ���̼��ԭ����___��
![]()
��2���̲IJ�ͼ���м������ں��ḻ���ص㡣��ش��������⣺
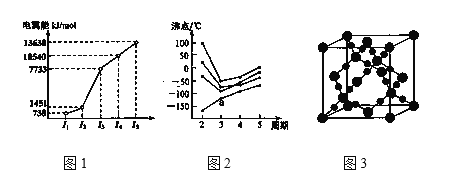
�ٵ������ڵ�ij����Ԫ�أ����һ�����������������ͼ1��ʾ�����Ԫ�ض�Ӧ��ԭ����___�ֲ�ͬ�˶�״̬�ĵ��ӡ�
��CO2�ڸ��¸�ѹ�����γɵľ����侧����ͼ3��ʾ����þ������������___���塣
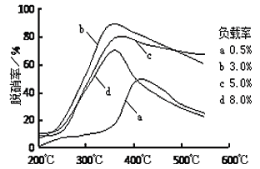
����ͼ2��ʾ��ÿ�����߱�ʾ���ڱ���A����A �е�ijһ��Ԫ���⻯��ķе�仯��ÿ��С�ڵ����һ���⻯�����a���������__���ж�����___��
���𰸡�1s22s22p63s23p63d64s2 sp3 ���� �ڷ�Ӧ�����У����Ӵ������ϸߵĹ��Ǩ�Ƶ������ϵ͵Ĺ�����Թ����ʽ�ͷ����� Mn2+��3d��������Ų�Ϊ�����״̬�����ȶ� ������ sp3 S8��CS2���ǷǼ��Է��ӣ�������������ԭ��֪S8������CS2 12 ԭ�Ӿ��� SiH4 �������嵽�������������Ԫ�ص��⻯���У�ֻ�е��������Ԫ���⻯��е㲻���ڷ���������aΪ�������ڵ��⻯���aΪSiH4��
��������
��ͼ���֪aΪH��bΪLi��cΪC��dΪN��eΪO��fΪF��gΪNa��hΪMg��iΪAl��jΪSi��kΪS��lΪCl��mΪAr��nΪK���ݴ˽��з�����
��1��pΪ26��Ԫ�أ������������ԭ����д�����Ų�ʽ��
��2����������ԭ��Nԭ�ӵļ۲���Ӷ����ж�̼ԭ�ӵ��ӻ���ʽ��
��3��h�ĵ�����Mg���ڿ�����ȼ�շ���ҫ�۵İ⣬�ڷ�Ӧ�����У����Ӵ������ϸߵĹ��Ǩ�Ƶ������ϵ͵Ĺ�����Թ����ʽ�ͷ�������
��4����Mn2+ת��ΪMn3+ʱ��3d�ܼ��ɽ��ȶ���3d5�����״̬ת��Ϊ���ȶ���3d4״̬����Fe2+ת��ΪFe3+ʱ��3d�ܼ��ɲ��ȶ���3d6ת��Ϊ���ȶ���3d5�����״̬��
II.��1����̬Sԭ�Ӻ�������Ų�ʽΪ1s22s22p63s23p4��Sԭ���γ�2��S-S��������2�Թµ��Ӷԣ��ӻ������Ϊ4��S8��CS2���ǷǼ��Է��ӣ��������ܣ�
��2���ٸ�����Ԫ�ص�һ���ڶ������ܽ�С�������������ܾ�����˵��������ʧȥ2�����ӣ��������2�����ӣ��ִ��ڵ������ڣ���ΪMg��ԭ�Ӻ���û���˶�״̬��ͬ�ĵ��ӣ�
��C��Oԭ��֮��ͨ�����ۼ��γɿռ�������״�ṹ��
�۵������嵽�������������Ԫ�ص��⻯���У�NH3��H2O��HF�ķ���֮�������������ǵķе���ͬ��������Ԫ�ص��⻯������쳣�ĸߣ�ͼ��a��û���쳣��˵��Ϊ���������Ԫ�ص��⻯�a������������ڵ��⻯�
��1��pΪ26��FeԪ�أ���̬ԭ�ӵ����Ų�ʽΪ1s22s22p63s23p63d64s2;
��2��d��a��Ӧ�IJ���NH3�������е�ԭ�Ӽ۲���Ӷ���Ϊ3+![]() =4������Nԭ�ӵ��ӻ���ʽΪsp3�ӻ�����ԭ�ӵŶԵ�����Ϊ1���ռ�ṹΪ���ԳƵ������Σ�Ϊ���Է��ӣ�
=4������Nԭ�ӵ��ӻ���ʽΪsp3�ӻ�����ԭ�ӵŶԵ�����Ϊ1���ռ�ṹΪ���ԳƵ������Σ�Ϊ���Է��ӣ�
��3��h�ĵ�����Mg���ڿ�����ȼ�շ���ҫ�۵İ⣬�ڷ�Ӧ�����У����Ӵ������ϸߵĹ��Ǩ�Ƶ������ϵ͵Ĺ�����Թ����ʽ�ͷ�������
��4����Mn2+ת��ΪMn3+ʱ��3d�ܼ��ɽ��ȶ���3d5�����״̬ת��Ϊ���ȶ���3d4״̬����Ҫ�������϶࣬��Fe2+ת��ΪFe3+ʱ��3d�ܼ��ɲ��ȶ���3d6ת��Ϊ���ȶ���3d5�����״̬��Ҫ���������٣�
II.��1����̬Sԭ�Ӻ�������Ų�ʽΪ1s22s22p63s23p4������ܼ��ĵ���Ϊ3p���ӣ�������Ϊ�����Σ�Sԭ���γ�2��S-S��������2�Թµ��Ӷԣ��ӻ������Ϊ4��Ϊsp3�ӻ���S8��CS2���ǷǼ��Է��ӣ�������������ԭ��֪S8������CS2��
��2���ٸ�����Ԫ�ص�һ���ڶ������ܽ�С�������������ܾ�����˵��������ʧȥ2�����ӣ��������2�����ӣ��ִ��ڵ������ڣ���ΪMg��ԭ�Ӻ���û���˶�״̬��ͬ�ĵ��ӣ��������12���˶�״̬��ͬ�ĵ��ӣ�
��C��Oԭ��֮��ͨ�����ۼ��γɿռ�������״�ṹ������þ���Ϊԭ�Ӿ��壻
�۵������嵽�������������Ԫ�ص��⻯���У�NH3��H2O��HF�ķ���֮�������������ǵķе���ͬ��������Ԫ�ص��⻯������쳣�ĸߣ�ͼ��a��û���쳣��˵��Ϊ���������Ԫ�ص��⻯�a������������ڵ��⻯�a���������SiH4��