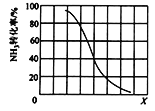
��Ŀ����
����Ŀ���ױ�(![]() )��һ����Ҫ�Ļ���ԭ�ϣ���������������ȩ(
)��һ����Ҫ�Ļ���ԭ�ϣ���������������ȩ(![]() )��������(
)��������(![]() )�Ȳ�Ʒ���±��г����й����ʵIJ����������ʣ���ش�
)�Ȳ�Ʒ���±��г����й����ʵIJ����������ʣ���ش�
���� | ��״ | �۵�(��) | �е�(��) | ����ܶ�(��ˮ��1g��cm��3) | �ܽ��� | |
ˮ | �Ҵ� | |||||
�ױ� | ��ɫҺ����ȼ�ӷ� | ��95 | 110.6 | 0.8660 | ���� | ���� |
����ȩ | ��ɫҺ�� | ��26 | 179 | 1.0440 | �� | ���� |
������ | ��ɫƬ״����״���� | 122.1 | 249 | 1.2659 | �� | ���� |
ע���ױ�������ȩ�������������ܡ�
ʵ���ҿ�����ͼװ��ģ���Ʊ�����ȩ��ʵ��ʱ��������ƿ�м���0.5g��̬�����Դ������ټ���15mL������(��Ϊ�ܼ�)��2mL�ױ�������������70�棬ͬʱ��������12mL�������⣬�ڴ��¶��½��跴Ӧ3Сʱ��
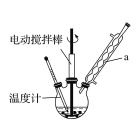
(1)װ��a��������______________��Ϊʹ��Ӧ��ϵ���ȱȽϾ��ȣ���________��
(2)����ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ_______________________________________________________��
(3)д������ȩ��������Һ��һ���������·�����Ӧ�Ļ�ѧ����ʽ��________________________________��
(4)��Ӧ��Ϻ�Ӧ���Һ������Ȼ��ȴ������ʱ����Ӧ����___________��__________(���������)�Ȳ��������ܵõ�����ȩ�ֲ�Ʒ��
(5)ʵ���м���������������ҷ�Ӧʱ��ϳ�����ʹ����ȩ��Ʒ�в����϶�ı����ᡣ����ӻ��б�����ı���ȩ�з���������ᣬ��ȷ�IJ���������______________(������˳������ĸ)��
a���Ի��Һ���з�Һ b�����ˡ�ϴ�ӡ�����
c��ˮ���м����������pH��2 d����������̼��������Һ�����
���𰸡����������� ˮԡ���� ![]() ��2H2O2
��2H2O2 ![]()
![]() ��3H2O
��3H2O 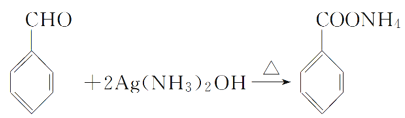 +2Ag����H2O��3NH3 ���� ���� dacb
+2Ag����H2O��3NH3 ���� ���� dacb
��������
��1��װ��a�����������������ܡ�ˮԡ���ȿ�ʹ��Ӧ��ϵ���ȱȽϾ��ȣ�
��2���ױ���H2O2��Ӧ���ɱ���ȩ��ˮ��������ƿ�з�����Ӧ�Ļ�ѧ����ʽΪ��. ![]() ��2H2O2
��2H2O2 ![]()
![]() ��3H2O��
��3H2O��
��3������ȩ��������Һ�ڼ��������·�����Ӧ���ɱ�����李�ˮ�������ʺͰ�����
��ѧ����ʽΪ��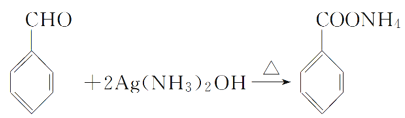 +2Ag����H2O��3NH3��
+2Ag����H2O��3NH3��
��4��ʵ��ʱ��������ƿ�м���0.5g��̬�����Դ����������ȹ��˳�ȥ��̬�����Դ����������ᡢ�ױ����������⡢����ȩ���ܣ��������Ƿе���죬��������ķ����õ�����ȩ�ֲ�Ʒ��
��5������ӻ��б�����ı���ȩ�з���������ᣬ���ȼ�������̼��������Һ�������������̼�����Ʒ�Ӧ���ɱ������ơ���������������ˮ������ȩ����ˮ���ٶԻ��Һ���з�Һ�����뿪����������Һ�ͱ���ȩ��Ȼ����ˮ���м����������pH��2���������ƺ����ᷴӦ���ɱ����ᣬ�����ˡ�ϴ�ӡ����ﱽ���ᣬ��Ϊdacb��