��Ŀ����
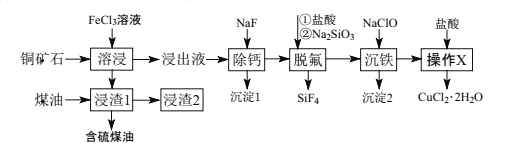
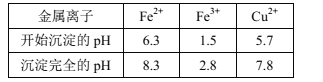
����Ŀ��þ�����������仯�������������������й㷺��Ӧ�á�
��1��þ���Ͻ����ڷɻ�����ҵ������3��90��þ���Ͻ�����������2mol/Lϡ����������0��2mol���������㲢ȷ��þ���Ͻ������ʵ���n(Mg): n(Al)=________��
��2�����������Ҫ�ɷ�ΪFeS2(��������ֻ��SiO2)�����������ԭ�ϡ�ȡij������10g�������Ŀ��������գ�4FeS2+11O2=2Fe2O3+8SO2������ַ�Ӧ����ȴ���Ƶù�������Ϊ7��4g������SiO2����Ӧ��������������FeS2����������Ϊ_____��
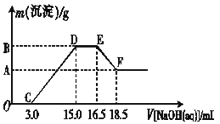
��3������һ���������ۺ�������ɵĻ�����100 mLϡ�����ַ�Ӧ����Ӧ���������κ�����ų�������Ӧ��ij�����Һ������4��00 mol��L��1��NaOH��Һ������NaOH��Һ���������������������Ĺ�ϵ��ͼ��ʾ(��Ҫʱ�ɼ��ȣ�����������ˮ�е��ܽ�)����������A�����ֵ��________��
��4���������������������������֡�ȡij������ĩ28��12g(����ֻ��Fe��C)�����������г�ַ�Ӧ���õ�CO2����224mL(��״����)��
������˸�����ĩ������̼�����ʵ���֮��Ϊ________________����������ȣ���
����ȡ���ݲ�ͬ����������������ĩ�ֱ�ӵ�100mL��ͬŨ�ȵ�ϡH2SO4�У���ַ�Ӧ��õ�ʵ���������±���ʾ��
ʵ����� | �� | �� | �� |
���������ĩ��������g�� | 2��812 | 5��624 | 8��436 |
��������������L������״���� | 1��120 | 2��240 | 2��800 |
���������Һ�����ʵ���Ũ��Ϊ__________________��
����������ʵ�����м�������m�˸�����ĩ�����㷴Ӧ������ʣ��Ĺ�������Ϊ___g(����3λС��)��
���𰸡�1:2 78% 0��856 50��1 1��25 mol/L (m��5��624)��![]() g
g
��������
��1����þ���������ʵ����ֱ�Ϊxmol��ymol�������ݻ�ѧ����ʽ������ɵ�24x+27y=3.9�٣�2x+3y=0.4�ڣ��ɴ˼���þ���Ͻ������ʵ�����
��2���������֪��Ӧǰ�������������10g-7.4g=2.6g����FeS2������Ϊx�����ɻ�ѧ����ʽ�ɵù�ϵʽ480��160=x��2.6g�����x=7��8���ɴ˼�����������FeS2������������
��3���������֪�����ۺ�������ɵĻ�����100 mLϡ�����ַ�Ӧ������������������������泥���ͼ��֪��OC��Ϊ����������������Һ��Ӧ��CD��Ϊ��������������������������Һ��Ӧ����������������������������DE��Ϊ�����������������Һ��Ӧ��EF��Ϊ����������������������Һ����ƫ�����ƣ����յõ���������������
��4���ٱ�״����224mL ������̼�����ʵ���Ϊ![]() =0��01mol����̼ԭ�Ӹ����غ��֪̼�����ʵ���0��01mol���ɸ�����ĩ����Ϊ28��12g��֪�������ʵ���Ϊ
=0��01mol����̼ԭ�Ӹ����غ��֪̼�����ʵ���0��01mol���ɸ�����ĩ����Ϊ28��12g��֪�������ʵ���Ϊ![]() =0��5(mol)���ɴ˼��������ĩ������̼�����ʵ���֮�ȣ�
=0��5(mol)���ɴ˼��������ĩ������̼�����ʵ���֮�ȣ�
���ɱ���֪������������ɷ�Ӧ�������������������������ʵ���Ũ�ȣ�
�۶Ա�ʵ��I��III��ȷ��������ĩ�е���ȫ���ܽ⣬�ɴ˼��㷴Ӧ������ʣ������������
��1����þ���������ʵ����ֱ�Ϊxmol��ymol�������ݻ�ѧ����ʽ������ɵ�24x+27y=3.9�٣�2x+3y=0.4�ڣ���٢ڿɵ�x=0��05��y=0��1����þ���Ͻ������ʵ���n(Mg): n(Al)= 0��05: 0��1=1:2���ʴ�Ϊ��1:2��
��2���������֪��Ӧǰ�������������10g-7.4g=2.6g����FeS2������Ϊx�����ɻ�ѧ����ʽ�ɵù�ϵʽ480��160=x��2.6g�����x=7��8����������FeS2����������Ϊ![]() ��100%/10=78%���ʴ�Ϊ��78%��
��100%/10=78%���ʴ�Ϊ��78%��
��3���������֪�����ۺ�������ɵĻ�����100 mLϡ�����ַ�Ӧ������������������������泥���ͼ��֪��OC��Ϊ����������������Һ��Ӧ��CD��Ϊ��������������������������Һ��Ӧ����������������������������DE��Ϊ�����������������Һ��Ӧ��EF��Ϊ����������������������Һ����ƫ�����ƣ����յõ�����������������ͼ��֪���������ܽ���������������Һ�����Ϊ��18.5��16.5��ml=2ml����3 NaOH��Al��OH��3��NaOH��NaAlO2��֪����������������������Һ�����Ϊ6ml���������������������������������������Ƶ����Ϊ��15.0��3.0��ml=12.0ml���������������������������Ƶ����Ϊ��12.0��6.0��ml=6.0ml����3 NaOH��Fe��OH��3��֪m[Fe��OH��3]=4.0mol/L��0.0060L��![]() ��107g/mol=0��856g����A�����ֵ��0��856���ʴ�Ϊ��0��856��
��107g/mol=0��856g����A�����ֵ��0��856���ʴ�Ϊ��0��856��
��4���ٱ�״����224mL ������̼�����ʵ���Ϊ![]() =0��01mol����̼ԭ�Ӹ����غ��֪̼�����ʵ���0��01mol���ɸ�����ĩ����Ϊ28��12g��֪�������ʵ���Ϊ
=0��01mol����̼ԭ�Ӹ����غ��֪̼�����ʵ���0��01mol���ɸ�����ĩ����Ϊ28��12g��֪�������ʵ���Ϊ![]() =0��5(mol)����˸�����ĩ������̼�����ʵ���֮��Ϊ0��5mol: 0��01mol=50��1���ʴ�Ϊ��50:1��
=0��5(mol)����˸�����ĩ������̼�����ʵ���֮��Ϊ0��5mol: 0��01mol=50��1���ʴ�Ϊ��50:1��
���ɱ���֪�������������״����2��800L���������ʵ���Ϊ![]() = 0��125mol����H2SO4��H2��֪������Һ�����ʵ���Ũ��Ϊ
= 0��125mol����H2SO4��H2��֪������Һ�����ʵ���Ũ��Ϊ![]() =1��25mol/L���ʴ�Ϊ��1��25mol/L��
=1��25mol/L���ʴ�Ϊ��1��25mol/L��
����ǡ���ܽ�ʱ����������Ϊx������ʵ��I��III�����¹�ϵ��![]() �����x��7��030g�������ĸ���������Ϊm��7��030��5��624��1��406g������������ĩ�е���δȫ���ܽ�ʱ����m��1��406g��˵��������ĩ�е���ȫ���ܽ⣬��Ӧ������ʣ����������Ϊ (m��5��624)��
�����x��7��030g�������ĸ���������Ϊm��7��030��5��624��1��406g������������ĩ�е���δȫ���ܽ�ʱ����m��1��406g��˵��������ĩ�е���ȫ���ܽ⣬��Ӧ������ʣ����������Ϊ (m��5��624)��![]() g=(m��5��624)��
g=(m��5��624)��![]() g�����ʴ�Ϊ��(m��5��624)��
g�����ʴ�Ϊ��(m��5��624)��![]() g����
g����