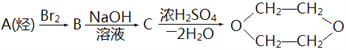̀âÄ¿ÄÚÈƯ
¡¾̀âÄ¿¡¿Ä³¿ÎÍâ»î¶¯Đ¡×éÀûÓĂÏÂͼװÖĂ½øĐĐ̉̉´¼µÄ´ß»¯Ñơ»¯ÊµÑé²¢ÖÆÈ¡̉̉È©£¬Í¼ÖĐ̀ú¼Ǜ¨µÈ×°ÖẲÑÂÔÈ¥£¬´ÖºÚÏß±íʾÈ齺¹Ü¡£Çë̀îĐ´ÏÂÁĐ¿Ơ°×£º
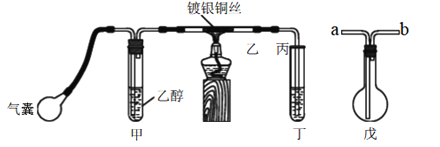
£¨1£©¼××°ÖĂ³£³£½₫ÔÚ70¡«80¡æµÄˮԡÖĐ£¬Ä¿µÄÊÇ____________________¡£
£¨2£©ÊµÑéʱ£¬ÏȼÓÈȲ£Á§¹Ü̉̉ÖеĶÆ̉øÍË¿£¬Ô¼1·ÖÖÓºó¹ÄÈë¿ƠÆø£¬´ËʱÍË¿¼´³Êº́ÈÈ×´̀¬¡£Èô°Ñ¾Æ¾«µÆ³·×ߣ¬¿ØÖÆ̉»¶¨µÄ¹ÄÆøËٶȣ¬ÍË¿Äܳ¤Ê±¼ä±£³Öº́ÈÈÖ±µ½ÊµÑé½áÊø¡£̉̉´¼µÄ´ß»¯Ñơ»¯·´Ó¦ÊÇ________·´Ó¦£῭î¡°·ÅÈÈ¡±»̣¡°ÎüÈÈ¡±£©£¬¸Ă·´Ó¦µÄ»¯Ñ§·½³̀ʽΪ______________¡£
£¨3£©ÈôÊԹܶ¡ÖĐÓĂË®ÎüÊƠ²úÎỘ̉ªÔÚµ¼¹Ü̉̉¡¢±ûÖ®¼ä½ÓÉÏÎ́×°ÖĂ£¬ÆäÁ¬½Ó·½·¨ÊÇ£῭îÎ́×°ÖĂÖе¼¹Ü´úºÅ£©£º̉̉½Ó_______¡¢_______½Ó±û£»Èô²úÎï²»ÓĂË®ÎüÊƠ¶øÊÇÖ±½ÓÀäÈ´£¬Ó¦½«ÊԹܶ¡½₫ÔÚ___________ÖĐ¡£
¡¾´đ°¸¡¿Ê¹Éú³É̉̉´¼ƠôÆøµÄËÙÂʼӿ́ ·ÅÈÈ 2CH3CH2OH+O2 ![]() 2CH3CHO+2H2O b a ±ùË®
2CH3CHO+2H2O b a ±ùË®
¡¾½âÎö¡¿
(1)¡¢¼ÓÈÈʱ²ÉÓĂˮԡ¼ÓÈÈ¿É̉ÔÈẲ̉´¼Æ½ÎÈÆû»¯³É̉̉´¼ƠôÆø£»
(2)¡¢¸Ă·´Ó¦̉ư·¢ºó£¬²»Đè¼ÓÈȼ´¿É½øĐĐ£¬ËµĂ÷·´Ó¦ÊÇ·ÅÈȵģ»̉̉´¼´ß»¯Ñơ»¯¿É̉ÔÉú³É̉̉È©£»
(3)¡¢°²È«Æ¿Öеĵ¼¹ÜÊÇ¡°¶̀½ø³¤³ö¡±£»ÀäÈ´ÎïÖỂ»°ă³£ÓñùË®»́ºÏÎïÀ´ÀäÈ´¡£
(1)¡¢̉̉´¼¾ßÓлӷ¢ĐÔ£¬Éư¸ßζÈÄÜ´Ù½ø̉̉´¼µÄ»Ó·¢£¬¼××°ÖĂ³£³£½₫ÔÚζÈΪ70~80µÄˮԡÖĐ£¬Ë®Ô¡ÄÜʹÈƯÆ÷ÊÜÈȾùÔÈ£¬ÄÜʹ¼×ÖĐ̉̉´¼Æ½ÎÈÆø»¯³É̉̉´¼ƠôÆø£¬
¹Ê´đ°¸Îª£ºÊʵ±¼Ó¿́Éú³É̉̉´¼ƠôÆøµÄËÙÂÊ£»
(2)¡¢ÊµÑéʱ£¬ÏȼÓÈȲ£Á§¹Ü̉̉ÖеĶÆ̉ø¸ÖË¿£¬Ô¼1·ÖÖÓºó¹ÄÈë¿ƠÆø£¬´ËʱÍË¿¼´³Êº́ÈÈ×´̀¬£¬Èô°Ñ¾Æ¾«µÆ³·×ߣ¬¿ØÖÆ̉»¶¨µÄ¹ÄÆøËٶȣ¬ÍË¿Äܳ¤Ê±¼ä±£³Öº́ÈÈÖ±µ½ÊµÑé½áÊø£¬ËµĂ÷·´Ó¦̉ư·¢ºó£¬²»Đè¼ÓÈȼ´¿É½øĐе½µ×£¬ËµĂ÷¸Ă·´Ó¦ÊÇ·ÅÈȵķ´Ó¦£»̉̉´¼·¢Éú´ß»¯Ñơ»¯2CH3CH2OH+O2 ![]() 2CH3CHO+2H2OÉú³É̉̉È©ºÍË®£»
2CH3CHO+2H2OÉú³É̉̉È©ºÍË®£»
¹Ê´đ°¸Îª£º·ÅÈÈ£»2CH3CH2OH+O2 ![]() 2CH3CHO+2H2O£»
2CH3CHO+2H2O£»
(3)¡¢Îª·ÀÖ¹¶¡ÖĐË®µ¹Îü£¬°²È«Æ¿Öеĵ¼Æø¹ÜÊÇ¡°¶̀½ø³¤³ö¡±£¬Ëù̉ÔÎ́×°ÖĂÓĐ»º³å×÷ÓĂ£¬ÀäÈ´ÎïÖỂ»°ă³£ÓñùË®»́ºÏÎïÀ´ÀäÈ´£»
¹Ê´đ°¸Îª£ºb£» a£»±ùË®¡£

¡¾̀âÄ¿¡¿Ä¿Ç°ÊÀ½çÉÏ60%µÄĂ¾ÊÇ´Óº£Ë®ÖĐ̀áÈ¡µÄ¡£̉ÑÖªº£Ë®̀áÈ¡Ă¾µÄÖ÷̉ª²½ÖèÈçͼ£º
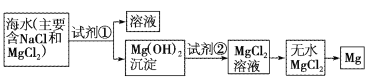
£¨1£©¹ØÓÚ¼ÓÈëÊÔ¼Á¢Ù×÷³Áµí¼Á£¬ÓĐ̉Ôϼ¸ÖÖ²»Í¬·½·¨£¬ÇëÍê³ÉÏÂÁĐÎỀâ¡£
·½·¨ | ÊÇ·ñƠưÈ· | ¼̣ÊöÀíÓÉ |
·½·¨1£ºÖ±½ÓÍùº£Ë®ÖĐ¼ÓÈë³Áµí¼Á | ²»ƠưÈ· | º£Ë®ÖĐĂ¾Àë×ÓŨ¶ÈĐ¡£¬³Áµí¼ÁµÄÓĂÁ¿´ó£¬²»¾¼Ă |
·½·¨2£º¸ßμÓÈÈƠô·¢º£Ë®ºó£¬ÔÙ¼ÓÈë³Áµí¼Á | ²»ƠưÈ· | £¨̉»£© |
ÄăÈÏΪ×îºÏÀíµÄÆäËû·½·¨ÊÇ£º£¨¶₫£© | ||
£¨̉»£©___£»
£¨¶₫£©___£»
£¨2£©¿̣ͼÖĐ¼ÓÈëµÄÊÔ¼Á¢ÙÓ¦¸ĂÊÇ___£῭ѧʽ£©£»¼ÓÈëµÄÊÔ¼Á¢ÚÊÇ___£῭ѧʽ£©£»¹¤̉µÉÏÓÉÎ̃Ë®MgCl2ÖÆÈ¡Ă¾µÄ»¯Ñ§·½³̀ʽΪ___¡£
£¨3£©¼ÓÈëÊÔ¼Á¢Ùºó£¬Äܹ»·ÖÀëµĂµ½Mg(OH)2³ÁµíµÄ·½·¨ÊÇ___¡£