��Ŀ����
12��ȡ200mL��Na2CO3��Na2SO4�����Һ������һ��Ũ��Ba��OH��2��Һ200mL��ǡ����ȫ��Ӧ�����ˡ������õ�14.51g��ɫ��������Һ����Һ���Ϊ400mL�������ù���ϡ���ᴦ�������������ٵ�4.66g����������ų������㣺��1��ԭ���Һ��Na2SO4�����ʵ���Ũ��Ϊ0.1 mol•L-1��
��2�������������ڱ�״���µ����Ϊ1.12L��
��3�����˺�������Һ�����ʵ���Ҫ�ɷ�ΪNaOH�������ʵ���Ũ��Ϊ0.35 mol•L-1��
���� ���ˡ������õ�14.51g��ɫ����Ϊ���ᱵ��̼�ᱵ����������������ϡ���ᴦ���������������ٵ�4.66g�������ᱵΪ4.66g��̼�ᱵ����Ϊ14.51g-4.66g=9.85g��
��1������n=$\frac{m}{M}$�������ᱵ�����ʵ���������������غ���n��Na2SO4��=n��BaSO4�����ٸ���c=$\frac{n}{V}$����ԭ���Һ��Na2SO4�����ʵ���Ũ�ȣ�
��2������n=$\frac{m}{M}$����̼�ᱵ�����ʵ��������ɵ�����Ϊ������̼������̼Ԫ���غ���n��CO2��=n��BaCO3�����ٸ���V=nVm���������̼�������
��3�����˳�14.51g��������Һ������ΪNaOH�������������غ�n��NaOH��=2n��Na2SO4��+2n��Na2CO3�����ٸ���c=$\frac{n}{V}$����ԭ���Һ��NaOH�����ʵ���Ũ�ȣ�
��� �⣺���ˡ������õ�14.51g��ɫ����Ϊ���ᱵ��̼�ᱵ����������������ϡ���ᴦ���������������ٵ�4.66g�������ᱵΪ4.66g��̼�ᱵ����Ϊ14.51g-4.66g=9.85g��
��1������������غ���n��Na2SO4��=n��BaSO4��=$\frac{4.66g}{233g/mol}$=0.02mol��ԭ���Һ��Na2SO4�����ʵ���Ũ��Ϊ��$\frac{0.02mol}{0.02L}$=0��mol/L��
�ʴ�Ϊ��0.1��
��2������̼Ԫ���غ���n��CO2��=n��BaCO3��=$\frac{9.85g}{197g/mol}$=0.05mol����V��CO2��=0.05mol��22.4L/mol=1.12L��
�ʴ�Ϊ��1.12��
��3�����˳�14.51g��������Һ������ΪNaOH�������������غ�n��NaOH��=2n��Na2SO4��+2n��Na2CO3��=2��0.05mol+2��0.02mol=0.14mol����Ϻ���Һ�����Ϊ0.4L��������Һ��NaOH�����ʵ���Ũ��Ϊ��$\frac{0.14mol}{0.4L}$=0.35mol/L��
�ʴ�Ϊ��NaOH��0.35��
���� ���⿼��������йؼ��㣬��Ŀ�Ѷ��еȣ���ȷ�����ķ�Ӧ�ǽ���Ĺؼ���ע�������غ㷨���㣬�����ֿ�����ѧ���ķ�����������ѧ����������

| A�� | ԭ������X��Y | B�� | ԭ�Ӱ뾶X��Y | ||
| C�� | X��Yһ��ͬ���� | D�� | X�ǵڢ�A��Ԫ�أ�Y�ǵ�V��A��Ԫ�� |
| A�� | A����Է�������С��C��D����Է�������������B����Է������� | |
| B�� | ��AΪ2-�����������ʵ������ͼ������ת�� | |
| C�� | ��A��B��D��Ϊ��״�������CҲһ��Ϊ��״������ | |
| D�� | C��A�ķ�Ӧ����Ϊ�ӳɷ�Ӧ |
| A�� | 1mol����ȫ��Ӧ��һ��ʧȥ2NA������ | |
| B�� | 1mol�κ����ʶ�Լ��6.02��1023������ | |
| C�� | �����ʵ�����NO��NO2����Nԭ������Ϊ1mol | |
| D�� | ����£�22.4L����������ԭ������ΪNA |
| A�� | �ƺ���ˮ��Ӧ��2Na+2H2O=2Na++2OH-+H2�� | |
| B�� | ���������������FeO+2H+=Fe2++H2O | |
| C�� | FeCl2��Һ��Cl2��Ӧ��2Fe2++Cl2=2Fe3++2Cl- | |
| D�� | ̼������Һ��ʯ����ķ�Ӧ��CO32-+Ca2+=CaCO3�� |
| A�� | ��֬������ܶ࣬������ˮ���һ����һ������ͬ | |
| B�� | ���ۡ���ά�ض������࣬����ͨʽ��ͬ�������Dz���Ϊͬ���칹�� | |
| C�� | �ױ��������ڹ����·�Ӧ��Ҫ����2��4-���ȼױ� | |
| D�� | ֻ�����Ƶ�Cu��OH��2����Һ���Լ���������Һ����������Һ�͵�����Һ |
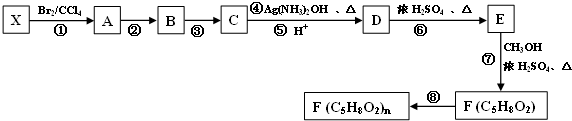
 ����̼�����ֻ��һ�����룬����һ��ֵ���ڴ����µ����ʽ��
����̼�����ֻ��һ�����룬����һ��ֵ���ڴ����µ����ʽ����1����CO2�뽹̿��������CO��CO�����������ȣ�
����֪��Fe2O3��s��+3C��ʯī���T2Fe��s��+3CO��g����H1=+489.0kJ/mol
C��ʯī��+CO2��g���T2CO��g����H2=+172.5kJ/mol����CO��ԭFe2O3���Ȼ�ѧ����ʽΪFe2O3��s��+3CO��g��=2Fe��s��+3CO2��g����H=-28.5kJ•mol-1��
���Ȼ��٣�PdCl2����Һ����Ӧ���ڼ���������CO��PdCl2����ԭ�ɵ��ʣ���Ӧ�Ļ�ѧ����ʽΪPdCl2+CO+H2O=Pd+CO2+2HCl��
��2��������ʯī�缫����KOH��Һ�У��������ֱ�ͨ��C3H8��O2�����ɱ���ȼ�ϵ�أ�
�ٸ����缫��Ӧʽ�ǣ�C3H8 +26OH--20e-=3CO32-+17H2O��
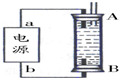
��ijͬѧ���ñ���ȼ�ϵ�������һ�ֵ�ⷨ��ȡFe��OH��2��ʵ��װ�ã���ͼ��ʾ����ͨ�����Һ�в��������İ�ɫ�������ҽϳ�ʱ�䲻��ɫ������˵������ȷ����ABD������ţ�
A����Դ�е�aһ��Ϊ������bһ��Ϊ����
B��������NaCl��Һ��Ϊ���Һ
C��A��B���˶������������缫
D�����������ķ�Ӧ�ǣ�2H++2e-=H2��
��3����һ���¶��£�������������һ����̼������Ӧ��Fe2O3��s��+3CO��g��?2Fe��s��+3CO2��g������֪�÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�ⳣ�������
| �¶�/�� | 1000 | 1150 | 1300 |
| ƽ�ⳣ�� | 64.0 | 50.7 | 42.9 |
����һ���ݻ�Ϊ10L���ܱ������У�1 000��ʱ����Fe��Fe2O3��CO��CO2��1.0mol����Ӧ����10min��ﵽƽ�⣮���ʱ�䷶Χ�ڷ�Ӧ��ƽ����Ӧ����v��CO2��=0.006mol•L-1��CO��ƽ��ת����Ϊ60%��
������ߢ���CO��ƽ��ת���ʣ��ɲ�ȡ�Ĵ�ʩ��C��
A������Fe���� B������Fe2O3���� C���Ƴ�����CO2 D����߷�Ӧ�¶� E����С�������ݻ� F��������ʵĴ���
��4����2.4g̼������������ȼ�գ���������ͨ��100mL 3.0mol/L������������Һ�У���ȫ���պ���Һ���������ӵ����ʵ���Ũ���ɴ�С��˳��Ϊc��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+����
| A�� | ��֬�������� | |
| B�� | ���ࡢ��֬�������ʶ��Ǹ߷��ӻ����� | |
| C�� | ú�ĸ�������к��з����� | |
| D�� | ������ȵ���ϩ�ͱ�ϩ��ȫȼ�����ĵ����������ʵ�����ͬ |
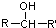 $\stackrel{[O]}{��}$
$\stackrel{[O]}{��}$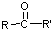 ��
��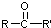 ���ױ�����������
���ױ�����������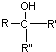 ���ױ�������ȩ��ͪ
���ױ�������ȩ��ͪ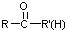 $\stackrel{HCN}{��}$
$\stackrel{HCN}{��}$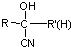 $\stackrel{H^{+}/H_{2}O}{��}$
$\stackrel{H^{+}/H_{2}O}{��}$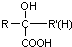 ��R��R�䡢R����������� ��
��R��R�䡢R����������� ��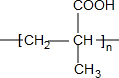 ��
�� ��
��