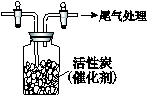��Ŀ����
��������(N2H4)�ǵ������ֳ���������ڿ�ѧ����������������Ҫ��Ӧ�á�����
����������м��㣺
�������������������ɵ�����һ���⻯����⻯�����Է�������Ϊ43.0�����е�
ԭ�ӵ���������Ϊ0.977������ȷ�����⻯��ķ���ʽΪ �����⻯����ײ������ȫ�ֽ�Ϊ������������4.30 g���⻯����ײ��������������ڱ�״���µ���� L��
��������������������������ƽ�����������ȼ�ϣ�����������������������Ӧ����
�ǵ�����ˮ����������������������ɵĻ���ƽ���ǡ����ȫ��Ӧ����72.0 kgˮ���ƽ���������������Ϊ kg��
����ˮ��Һ����������NO��NO2������壬����������������Ի�������Ⱦ�����
��д���йصķ�Ӧ����ʽΪ�� �� ��
��1��HN3��N3H (1��) 4.48(1��)
��2��64 kg (2��)
��3��4NH3+6NO=5N2+6H2O (2��) 8NH3+6NO2=7N2+12H2O (2��)��д��NH3�� H2OҲ���֣�
���������������1��N(N)=43.0��0.977��14=3��N(H)=(43.0-14��3)��1=1������ʽΪ HN3��
n(HN3)=4.30��43=0.1mol������ԭ���غ�õ�n(H2)=0.05mol��n(N2)=0.15mol
����������0.05+0.15����22.4=4.48L
��2���÷�Ӧ����ʽΪ2N2H4+N2O4=3N2+4H2O�����ݷ���ʽ����ɵ�m(N2H4)=64kg
��3��4NH3+6NO=5N2+6H2O 8NH3+6NO2=7N2+12H2O
���㣺����Ԫ�ػ����P��Ӧ�ȵļ����й����⡣

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д���ϴ�������ǹ辧Ƭ��������Ҫ����֮һ����Ƭ��ѧ��ϴ����ҪĿ���dz�ȥ��Ƭ�������ʣ���ijЩ�л�����Σ�������Si��SiO2�۳��ȣ������õĻ�ѧ��ϴ���иߴ�ˮ���л��ܼ���˫��ˮ��Ũ�ᡢǿ��ȡ�����ȥ����������ͨ����һ��Ũ�ȵ�HF��Һ�����������½���Ƭ����1�������ӡ��������ڹ�Ƭ�����γɽ������ε����棬���ӹ��̫��������ա���������ͨ����NaOH��Na2SiO3�Ȼ����Һ��75��90�淴Ӧ25��35 min��Ч�����á�
�ش���������
��1���ܷ��ò����Լ�ƿ��ʢHF��Һ��Ϊʲô?�û�ѧ����ʽ���Խ��� ��
��2��д����Ƭ����Ӧ�����ӷ���ʽ ���Ե�������1990�껯ѧ��Seidel�����һ�ֵĵ绯ѧģ�ͣ���ָ��Si��NaOH��Һ�ķ�Ӧ��������Si��OHһ��Ӧ������SiO44һ��Ȼ��SiO44һѸ��ˮ������H4SiO4�����ڴ�ԭ��������Ӧ��������Ϊ ��
��3����У��ѧ��ȤС��ͬѧ��Ϊ��֤Seidel�������Ƿ���ȷ���������ʵ�飺
| | ʵ����ʵ |
| ��ʵһ | ˮ������600��ʱ��ʹ��ĩ״�軺���������ų������� |
| ��ʵ�� | ʢ���ڲ���ʯӢ�����еĴ�ˮ��ʱ��Է�ĩ״��ԭ����ʴ���á� |
| ��ʵ�� | ��ͨ���������е�ˮ�����дӲ������ܳ������ļ���ʹ��ĩ״�������л����ܽ⡣ |
| ��ʵ�� | ��Ұ�����ýϸ߰ٷֱȵĹ�����������Ca(OH)2��NaOH�����ź����գ��ɾ��ҷų�H2�� |
| ��ʵ�� | 1g��0.036mo1��Si��20mL����lgNaOH��0.025mol������Һ��С�ļ��ȣ���Ԥ�ȣ����ռ���Լ1700mL H2���ܽӽ�����ֵ��1600mL���� |
���ۣ���ʵ����˵������ˮ��Һ�����£�H2O���� ����NaOH�� �������ͷ�Ӧ ��������ˮ�����£�NaOH�� ����
��4����̫���ܵ�ر����������ɫ����Ĥ���������辧Ĥ�����ù��飨SiH4���백����NH3���ڵ��������з�Ӧ��������һ����ɫ���ж����壬�������������ˮ���ҷ�Ӧ�����й��ڹ��顢���������������ȷ���� ��
A����ʹ�ù���ʱҪע����������ˮ��SiH4����ˮ����������ԭ��Ӧ����H2��
B�������백����Ӧ�Ļ�ѧ����ʽΪ��3SiH4+4NH3��Si3N4+12H2������Ӧ��NH3����������
C�����Ǿ���Խ�Ŀ���������Ե���ܺ������ܣ���ѧ�ȶ��Ժܺã������κ��ᡢ�Ӧ��
D�������辧����ֻ���ڹ��ۼ���Si3N4���������������ǽ������ϡ�
��16�֣������������ҹ�����Դ������ռ�ϴ���أ������ŷų���SO2�����һϵ�л�������̬���⣬ֱ���ŷź�SO2���������γ����꣬Σ��������
��1���û�ѧ����ʽ��ʾSO2�γ�����������ķ�Ӧ�� ��2��
��2����ҵ����Na2SO3��Һ���������е�SO2��������ͨ��1.0 mol��L-1��Na2SO3��Һ����ҺpH���ϼ�С������ҺpHԼΪ6ʱ������SO2�����������½���Ӧ�������ռ���
�� ��ʱ��Һ��c(SO32�C)��Ũ����0.2 mol��L-1������Һ��c(HSO3�C)��_______mol?L-1��
�� ��pHԼΪ6�����ռ���ͨ��������O2���ɽ����е�NaHSO3ת��Ϊ�������ʣ���Ӧ�Ļ�ѧ����ʽ�� ��2��
�� ij�о�С��Ϊ̽����ߺ���������SO2������Ч�ʵĴ�ʩ��ģ��ʵ�����պ���������ʵ������ͼ��ʾ���� �����������SO2������Ч�ʡ�2��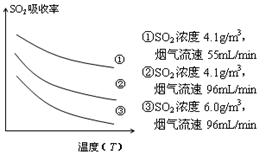
��3�������ֿ��ŵ�Na2SO3ҩƷ�Ѳ��ֱ������������û�ѧС��������֪Ũ�ȵ�����KMnO4��Һ��ȷ���京�������岽�����£�
����i����ȡ��Ʒ1.000 g��
����ii������Ʒ�ܽ����ȫת�Ƶ�250 mL����ƿ�У����ݣ����ҡ�ȡ�
����iii����ȡ25.00 mL��Ʒ��Һ��250 mL��ƿ�У���0.01000 mol��L��1 KMnO4����Һ�ζ����յ㡣
�����������������ظ�2�Ρ�
�� д������iii��������Ӧ�����ӷ���ʽ_________________________________��
�� ������0.01000 mol��L��1 KMnO4��Һʱ�����Ӷ��ݣ������ղ��ҩƷ��Na2SO3�ĺ���________(�ƫ����ƫС������Ӱ�족)��
�� ijͬѧ����������������еζ�ʵ��(�гֲ�����ȥ)�������������� (����ĸ)��

A B C D E
�� �ζ�������±���ʾ��
| �ζ����� | ������Һ �����/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25.00 | 1.02 | 21.03 |
| 2 | 25.00 | 2.00 | 21.99 |
| 3 | 25.00 | 2.20 | 20.20 |
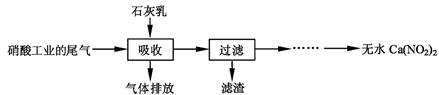
 N2O3(g)����ƽ�ⳣ������ʽΪK= ��
N2O3(g)����ƽ�ⳣ������ʽΪK= ��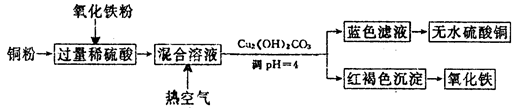

 ��
��