��Ŀ����
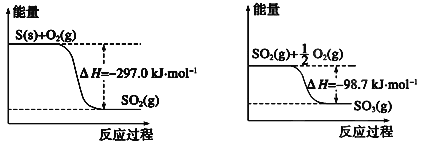
����Ŀ��CH3CH2CH2CH3��g����![]() O2��g��=4CO2��g����5H2O��l�� ��H����2878kJ/mol����CH3��2CHCH3��g����
O2��g��=4CO2��g����5H2O��l�� ��H����2878kJ/mol����CH3��2CHCH3��g����![]() O2��g��=4CO2��g����5H2O��l����H����2869kJ/mol������˵����ȷ����
O2��g��=4CO2��g����5H2O��l����H����2869kJ/mol������˵����ȷ����
A.���������춡���������С��ϵ��ͼ
B.��������ȶ��Դ����춡��
C.�춡��ת��Ϊ������Ĺ�����һ�����ȹ���
D.�춡������е�̼�����������Ķ�
���𰸡�A
��������
A�������춡��ת��Ϊ������������ߵ����жϣ�
B���������ʾ��е�����Խ��Խ�ȶ����жϣ�
C�������춡��ת��Ϊ������������ߵ����жϣ�
D��������������춡���к��еĻ�ѧ�����ش�
A���춡��ת��Ϊ������Ӧ���ȣ��������������춡��ߣ���A��ȷ��
B�����ʾ��е�����Խ��Խ�ȶ�����ͬ���ʵ�������������춡��ȼ�գ�������ų��������࣬�ɼ����춡��Ƚ��ȶ�����B����
C���������������춡��ߣ����ԣ��춡��ת��Ϊ������Ӧ���ȣ���C����
D���춡��������鶼����̼̼������̼������ɣ�������ѧ���������Ŀ��ͬ����D����
��ѡA��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ