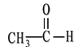��Ŀ����
����Ŀ��(1)���з�Ӧ�����ڼ��˾���Ƿ�ƺ��ʻ��2![]() +3CH3CH2OH+16H++13H2O
+3CH3CH2OH+16H++13H2O![]() 4[Cr(H2O)6]3++3CH3COOH
4[Cr(H2O)6]3++3CH3COOH
�������[Cr(H2O)6]3+�У���Cr3+�γ���λ����ԭ����___(��Ԫ�ط���)��
��CH3COOH��Cԭ�ӹ���ӻ�����Ϊ___��1 mol CH3COOH���Ӻ�����������ĿΪ___��
(2)CS2�����У�Cԭ�ӵ��ӻ����������____��д��������CS2������ͬ�ռ乹�ͺͼ�����ʽ������____��
(3)ʯīϩ��һ���ɵ���̼ԭ�ӹ��ɵ�ƽ��ṹ����̼���ϣ�ʯīϩ�в���̼ԭ�ӱ���������ƽ��ṹ�ᷢ���ı䣬ת��Ϊ����ʯīϩ��
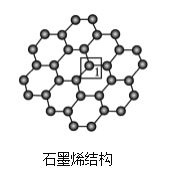
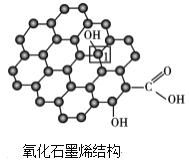
����ʯīϩ�У�1��C���ӻ���ʽ��____����C������C�γɵ�C-C����____(����>������<������=��)ʯīϩ����1��C����C�γɵ�C-C���ǡ�
���𰸡�O sp3��sp2 7 NA sp SCN- OCN-�� sp3 <
��������
(1)�����[Cr(H2O)6]3+��Cr3+Ϊ�������ӣ�H2OΪ���壻
(2)CH3COOH��Cԭ�ӷֱ��γ�4����3��������û�й¶Ե��ӣ�CH3COOH�����к���1��C-C��3��C-H��1��C-O��1��C=O��1��O-H�Ȼ�ѧ����
(3)CS2���ӵĽṹʽΪS=C=S����������¶Ե������жϼ۲���Ӷ������ж��ӻ����ͣ���CS2������ͬ�ռ乹�ͺͼ�����ʽ������Ϊ�ȵ����壬Ӧ����3��ԭ�ӣ��۵�����Ϊ16��
(4)����ʯīϩ��1��Cԭ���γ�4���������۲���ӶԸ�����4�����ݼ۲���ӶԻ��������ж�Cԭ���ӻ���ʽ������ʯīϩ��1��̼ԭ�Ӻ�3��C��1��Oԭ���γ�������ṹ��ʯīϩ��ÿ��Cԭ�Ӻ�����3��̼ԭ���γ�ƽ��ṹ���ݴ˷����жϡ�
(1)�������[Cr(H2O)6]3+��Cr3+Ϊ�������ӣ�H2OΪ���壬Oԭ���ṩ�¶Ե��ӣ���Cr3+�γ���λ�����ʴ�Ϊ��O��
��CH3COOH��Cԭ�ӷֱ��γ�4����3����������û�йµ��Ӷԣ��ֱ�Ϊsp3�ӻ���sp2�ӻ���CH3COOH�����к���1��C-C��3��C-H��1��C-O��1��C=O��1��O-H��ѧ������1mol CH3COOH�����к�����������ĿΪ7 NA��7��6.02��1023���ʴ�Ϊ��sp3��sp2��7 NA��7��6.02��1023��
(2)CS2������Cԭ���γ�2���������¶Ե�����Ϊ![]() =0����CΪsp�ӻ���
=0����CΪsp�ӻ���
��CS2������ͬ�ռ乹�ͺͼ�����ʽ������Ϊ�ȵ����壬Ӧ����3��ԭ�ӣ��۵�����Ϊ16��������SCN-��OCN-�ȣ��ʴ�Ϊ��sp��SCN-��OCN-�ȣ�
(3)����ʯīϩ�У�1��C�γ�3��C-C��1��C-O����Cԭ����sp3�ӻ���Ϊ�����幹�ͣ���ʯīϩ�е���1��C�γ�C-C���ĵ�Cԭ����sp2�ӻ���Ϊƽ�������ι��ͣ�������ʯīϩ��1��C������C�γɵ�C-C���ǣ�ʯīϩ����1��C����C������C�γɵ�C-C���ǣ��ʴ�Ϊ��sp3������