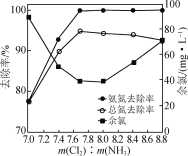
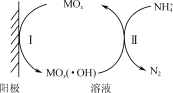
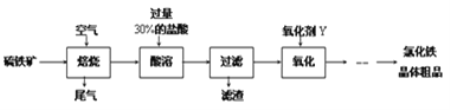
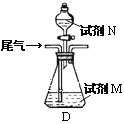
��Ŀ����
����Ŀ��ijѧ����0.1000mol/LKOH��Һ�ζ�δ֪Ũ�ȵ�������Һ��������ɷ�Ϊ���¼�����
(A)��ȡ20.00mL�����������Һע��ྻ����ƿ��������2-3�η�̪
(B)�ñ���Һ��ϴ�ζ���2-3��
(C)��ʢ��KOH����Һ�ļ�ʽ�ζ��̶ܹ��ã�����Һ��ʹ�ζ��ܼ��������Һ
(D)ȡ����Һע���ʽ�ζ�����0�̶�����2-3cm
(E)����Һ����0��0�̶����£����¶���
(F)����ƿ���ڵζ��ܵ����棬�ñ�KOH��Һ�ζ����յ㣬���µζ���Һ��Ŀ̶�
���������գ�
��1����ȷ������˳����(�������ĸ��д)B��__��__��__��__��F��
��2������(B)������Ŀ����___��
��3���жϵζ������յ��������___��
��4�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼ��ʾ������ʼ����Ϊ__mL���յ����Ϊ___mL������������Һ��Ũ��Ϊ___mol/L��

��5�����ζ��յ����ʱ�����ӵζ��ܵĿ̶ȣ�������������ȷ����Եζ����������Ӱ����__������ȡһ������KOH����(������NaOH)���Ʊ���Һ�������ζ��������ᣬ��Եζ����������Ӱ����___��(����ƫ��������ƫ������������Ӱ����)��
���𰸡�D C E A ��ֹϡ�ͱ�Һ ��ƿ����Һ����ɫ����ɫ��dz���ұ���30���ڲ��� 0.00 25.90 0.1295 ƫ�� ƫ��
��������
(1)�����к͵ζ��м�©��ϴ�ӡ���ϴ��װҺ��ȡ����Һ����ָʾ�����ζ��Ȳ�������
(2)�ζ�����װҺʱ��Ϊ�˷�ֹ��Һ��ϡ�ͣ����ô�װҺ��ϴ��
(3)����Һ��ɫ�仯�Ұ�����ڲ���ɫ����˵���ﵽ�ζ��յ㣻
(4)���ݵζ��ܵĽṹ�;�ȷ���Լ�������ԭ��������c(����)=![]() �������ҺŨ�ȣ�
�������ҺŨ�ȣ�
(5)����c(����)=![]() ����������������V(��)��Ӱ�죬�Դ��ж���
����������������V(��)��Ӱ�죬�Դ��ж���
(1)�к͵ζ����ռ�©��ϴ�ӡ���ϴ��װҺ��ȡ����Һ����ָʾ�����ζ���˳�����������ȷ��˳��ΪBDCEAF���ʴ�Ϊ��D��C��E��A��
(2)�ñ���Һ��ϴ�ζ���2-3�ε�Ŀ���Ƿ�ֹ����Һϡ�ͣ�Ӱ��ⶨ������ʴ�Ϊ����ֹϡ�ͱ�Һ��
(3)��0.1000mol/LKOH��Һ�ζ�δ֪Ũ�ȵ�������Һ������Һ��ɫ����ɫ��Ϊdz��ɫ���Ұ���Ӳ��ָ�����˵���ﵽ�ζ��յ㣬�ʴ�Ϊ����ƿ����Һ��ɫ����ɫ��dz��ɫ���Ұ���Ӳ���ɫ��
(4)�ζ���С�����ϣ��������£�����ͼʾ����ʼ����Ϊ0.00mL���յ����Ϊ25.90mL��������Һ�����Ϊ25.90mL�������ҺŨ��Ϊ��c=![]() =0.1295mol/L���ʴ�Ϊ��0.00��25.90��0. 1295mol/L��
=0.1295mol/L���ʴ�Ϊ��0.00��25.90��0. 1295mol/L��
(5)���ζ��յ����ʱ�����ӵζ��ܵĿ̶ȣ�������������ȷ���ᵼ�����ı�Һ�����ƫС������c(����)=![]() ��֪����ҺŨ��ƫ�ͣ�����ȡһ������KOH����(������NaOH)���Ʊ���Һ�������ζ��������ᣬ�������ı�Һ���С������c(����)=
��֪����ҺŨ��ƫ�ͣ�����ȡһ������KOH����(������NaOH)���Ʊ���Һ�������ζ��������ᣬ�������ı�Һ���С������c(����)=![]() ��֪����ҺŨ��ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�ƫ�͡�
��֪����ҺŨ��ƫ�ͣ��ʴ�Ϊ��ƫ�ͣ�ƫ�͡�

����Ŀ��NH3�����ζ�����Ҫ�Ļ���ԭ�ϡ�
��1����NH4Cl��Ca��OH��2�Ʊ�NH3����Ӧ�����������ռ���β������װ������Ϊ_____��
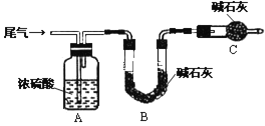
��2������ͼװ�ý���NH3����ʵ�顣
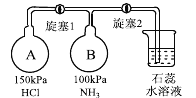
���ȴ�����1��Bƿ�е�������_______��ԭ����__________���ȶ��ر�����1��
���ٴ�����2��Bƿ�е�������_________________��
��3�����ʵ�飬̽��ijһ�����ض���Һ��NH4Clˮ��̶ȵ�Ӱ�졣
�����Լ�������������NH4Cl������ˮ��100mL����ƿ���ձ�����ͷ�ιܡ���������ҩ�ס���ƽ��pH�ơ��¶ȼơ�����ˮԡ�ۣ��ɿ����¶ȣ�
��ʵ��Ŀ�ģ�̽��______����Һ��NH4Clˮ��̶ȵ�Ӱ�졣
�����ʵ�鷽�����ⶨʵ�������������ʵ�鷽�����г���ֱ�Ӷ�ȡ���ݵ���������������ⶨ�����ݣ���������ĸ��ʾ������V����Һ����ʾ��������Һ���������_________________
������ | V����Һ��/mL | ���� | |||
1 | 100 | ||||
2 | 100 |
����ʵ�����I�������ݽ���ʵ�飬����ȡ�Ĵ�������������ֵΪY����NH4Clˮ�ⷴӦ��ƽ��ת����
Ϊ_____��ֻ�г���ʽ������ˮ���������Ӱ�죩��
����Ŀ��һ���¶��£�10mL0.40molL-1H2O2��Һ�������ֽ⡣��ͬʱ�̲������O2�����(������Ϊ��״��)���±���
t/min | 0 | 2 | 4 | 6 | 8 | 10 |
V(O2)/mL | 0.0 | 9.9 | 17.2 | 22.4 | 26.5 | 29.9 |
������������ȷ����(��Һ����仯���Բ���)�� ��
A.0~6min��ƽ����Ӧ���ʣ�v(H2O2)��3.3��10-2mol��L-1��min-1
B.6~10min��ƽ����Ӧ���ʣ�v(H2O2)<3.3��10-2mol��L-1��min-1
C.��Ӧ��6minʱ��c(H2O2)=0.30mol��L-1
D.��Ӧ��6minʱ��H2O2�ֽ���50%