��Ŀ����
����Ŀ��I. �����£���3��һԪ��ֱ��NaOH��Һ�������ϣ�ʵ���������£�
��� | c��һԪ�ᣩ��c��H+�� | c��NaOH����mol/L | �����Һ��pH |
�� | c��H+��=0.1 mol��L��HX�� | 0.1 | pH=a |
�� | c��HY��=0.1mol��L | 0.1 | pH=7 |
�� | c��HZ��=0.1 mol��L | 0.1 | pH=9 |
��1������ʵ����HXΪ���ᣬa______7���<������=����>������
��2������ʵ����HY��Һ��pH=______��
��3������ʵ�鷢����Ӧ�����ӷ���ʽΪ__________________��
������Һ����ˮ�������c��OH-��=______mol/L��
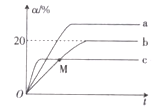
II. ��0.1 mol��L-1 NaOH��Һ�ֱ���������Ϊ20 mL��Ũ�Ⱦ�Ϊ0.1 mol��L-1 HCl��Һ��HA��Һ����Һ��pH�����NaOH��Һ����仯��ͼ��

��1��HAΪ_______�ᣨ�ǿ������������
��2��N��pH>7��ԭ���ǣ������ӷ���ʽ��ʾ��_________��
��3������˵����ȷ����_______ ������ĸ����
a. M��c��HA��-��
b. M��c��Na+��>c��OH-��
c. P��c��Cl-��=0.05 mol��L-1
��4��NaA��NaOH��Ũ�ȵ�������ʱ����Һ��c��A-��______c��OH-�����<������=����>����
���𰸡� < 1 HZ+OH-��H2O+Z- 10-5 �� A-+H2O![]() HA+OH- b <
HA+OH- b <
������������1��HX�����ᣬֻ�ܵ������һ���֣�����c��H+��=0.1 mol��L��HX��Һ�У�HX��Ũ��Զ����0.1 mol��L������Һ��0.1mol/L������������Һ�������ϣ�һ����HX�����ܶ࣬������Һ�����ԣ�a��7��
��2��HY��NaOH�������Ũ�Ȼ�ϣ�Ӧ��ǡ���к͵õ�NaY����Һ����Һ��pH=7������˵��HY��һԪǿ�ᣬ��0.1mol/L��HY��Һ��pH=1��
��3��HZ��NaOH�������Ũ�Ȼ�ϣ�Ӧ��ǡ���к͵õ�NaZ����Һ����Һ��pH=9������˵��HZ��һԪ���ᣬ������к͵ķ�ӦΪ��HZ+OH-��H2O+Z-��
II.��1��������ǿ�ᣬ0.1mol/L������pH=1�����Դ�pH=1�ĵ���������ߴ������ᡣ����һ��ΪHA��ͼ�п�����0.1mol/L��HA��Һ��pH=5������HA�����ᡣ
��2��N���������20mL��0.1mol/L������������Һ����ʱ���������������HAǡ����ȫ�кͣ��õ�NaA����Һ������Һ�Լ��Ե�ԭ����A-��ˮ�⣨HA�����ᣩ�������ӷ���ʽΪ��A-+H2O![]() HA+OH-��
HA+OH-��
��3��M���������10mL 0.1mol/L������������Һ����ʱ�к�һ���HA�����Եõ�c(HA)=c(NaA)�Ļ����Һ��ͼ�п�����Һ�Լ��ԣ�����HA�ĵ�������NaA��ˮ�⣬�õ���c(HA)��c(Na+)��c(A-)��c(OH-)��c(H+)������ѡ��a����ѡ��b��ȷ��P���ʾ��20 mL 0.1 mol��L-1 HCl��Һ�м���10mL 0.1mol/L������������Һ����ʱc��Cl-��=0.1��2/3=0.067 mol��L-1��ѡ��c����
��4��NaA��NaOH��Ũ�ȵ�������ʱ����ʼc��A-����c��OH-����A-ˮ������OH-������c��A-����c��OH-����

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�