��Ŀ����
��������Ҫ�Ļ�����Ʒ���ڻ�����ѧʵ���У�������Ҫ�����á�
��1�����ʵ���Ũ��Ϊ18��4mol/L,��������Ϊ0��98��Ũ��������ˮ����ʱ���������������½���0��87���ܶ�1��8g?cm-3������ʱ����ʧȥ���������� ��������Ϊ0��87����������ʵ���Ũ��Ϊ
��������λС������ͬ����50mL 18��4mol/L��Ũ������Ϊ�����ʱ��������ˮ___________g��
��2����ҵ���Ը����������ᣮ����Ϊԭ����ȡ�����[NH4Al(SO4)2��12H2O]������������Ӧԭ�����£����Ը����������ɷ�������ķ�Ӧ����
Al2O3 + 3H2SO4 �� Al2(SO4)3 + 3H2O����������
Al2(SO4)3 + H2SO4 + 2NH3 �� 2NH4Al(SO4)2����������
ij������ͬʱ��ȡ���������������ͨ����������������������ֲ�Ʒ�IJ���������ʹ�Ƶõ�������������������ʵ���֮��Ϊ1:1����Ͷ��ʱ����������������ʵ���֮����
��3�����Ṥҵ�ϴ���ýӴ��������ᣨ��������������������Ϊ20%����Ϊʹ���������ճ�֣���ͨ�����40%�Ŀ����������պ�¯����SO2���������Ϊ_____��
��4��������¯��������������ֱ������Ӵ��ң��������������5%��ͬ��ͬѹ�²ⶨ�����Լ���SO2��ת����
��1��(4��)15��98 mol��L-1 11��63 ��2��(2��) 3��10
��3��(3��) 0��11 ��4��(3��) 93%��
���������������1��C=n/v=1��8*0��87*1000/98*1=15��98 ��mol��L-1 ��
�⣺��������ˮ������Ϊx �������ʵ���������
18��4*50*0��98=(x+18��4*50)*0��87 x=116��3
��2���⣺��������������������ʵ���Ϊx
Al2O3 + 3H2SO4 �� Al2(SO4)3 + 3H2O����������
3/2 x 9x/2 3/2 x
Al2(SO4)3 + H2SO4 + 2NH3 �� 2NH4Al(SO4)2����������
1 1 2
x/2 x/2 x
����������������ʵ���֮����: 3/2 x:( 9x/2+ x/2)= 3��10
��3���⣺����������Ϊa
4FeS2+11O2=2Fe2O3+8SO2
11 8
a*20% 0��145a
SO2���������Ϊ��0��145a *100%/��1+40%��a*80%+ 0��145a =0��108 a ����Ϊ0��11 a
��4��2SO2+ O2="2" SO3 �SV
2 1 1
10%a 5%a
SO2��ת����Ϊ��10%a*100%/0��108 a=93%
���㣺�����Թ�ҵ����Ϊ���������黯ѧ�����֪ʶ��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д������������ֽ�����ĩA��B�ֱ���ͬŨ�ȵ�����ϡ���ᷴӦ��������+2�۽����Ȼ���䷴Ӧ�����ͼ��ʾ��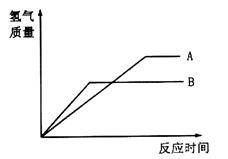
��1����Fe��Zn��Cu��A��__________��B��____________��
��2�������ֽ�����ĩ��һ��������Ϻ��мס��ҡ�������ʵ�飬����ʵ���ȡ500mLͬŨ�ȵ����������ֻ�Ϸ�ĩ���й��������£�
| ʵ����� | �� | �� | �� |
| ��Ϸ�ĩ����/g | 6��2 | 18��6 | 24��8 |
| ���������������״���£�/mL | 2240 | 5600 | 5600 |
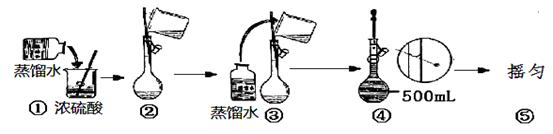
��������������������ʵ���Ũ�ȣ�д��������̣��С�
Ba2����һ���ؽ������ӣ���һ�������С��������Na2S2O3��KI��K2Cr2O7���Լ��ⶨij������ˮ��Ba2�������ʵ���Ũ�ȡ�
��1��������ƿ��ʹ�÷����У����в�������ȷ���ǣ�����ĸ��________��
| A��ʹ������ƿǰ������Ƿ�©ˮ |
| B������ƿ��ˮϴ�������ô�����Һ��ϴ |
| C��������Һʱ����������ǹ��壬�ѳƺõ�������ֽ��С�ĵ�������ƿ�У�������ˮ���ӽ�����1��2 cm�����õι���εμ�����ˮ������ |
| D��������Һʱ����������Һ�壬����Ͳ��ȡ������ֱ�ӵ�������ƿ�У�������ˮ���ӽ�����1��2 cm�����õι���εμ�����ˮ������ |
��2����������250 mL 0.100 mol��L��1�ı�Na2S2O3��Һ������Ҫ�IJ�����������Ͳ��250 mL����ƿ���������⣬����Ҫ________________��
��3����ȷ��ȡNa2S2O3���������Ϊ________g��
��4����ȡ��ˮ50.00 mL�������ʵ�����ȣ�����������K2Cr2O7��Һ���õ�BaCrO4������������ϴ�ӡ����˺���������ϡ�����ܽ⣬��ʱCrO42-ȫ��ת��ΪCr2O72-���ټ������KI��Һ���з�Ӧ��Ȼ���ڷ�ӦҺ�еμ�������Na2S2O3��Һ����Ӧ��ȫʱ������Na2S2O3��Һ36.00 mL����֪�йط�Ӧ�����ӷ���ʽΪ��Cr2O72-��6I����14H��=2Cr3����3I2��7H2O����I2��2S2O32-=2I����S4O62-����ù�����ˮ��Ba2�������ʵ���Ũ��Ϊ________��
����˵����ȷ����
| A������蘆��۵�ȵ���﮵ĸ� |
| B������Ľ�������ͨ����ֽ |
| C��Ԫ��N���⻯��ķе��Ԫ��P���⻯��ķе�� |
| D����пƬ����������Ƭ������������Ȼ�п��Һ����Ƭ�������һ��п |
 �����ʵ���Ũ�ȣ�
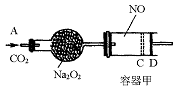
�����ʵ���Ũ�ȣ� ������CO2�ļ���ͨ�룬�������������ƶ����������ǻ�����ĥ����
������CO2�ļ���ͨ�룬�������������ƶ����������ǻ�����ĥ����